修回日期: 2009-07-22
接受日期: 2009-07-27
在线出版日期: 2009-08-18
目的: 观察褪黑素前干预对急性坏死性胰腺炎(ANP)大鼠的胰腺和肺组织的保护作用与硫氧还蛋白-1(Trx-1)表达的关系.
方法: 72只♂SD大鼠随机分成ANP模型组(A组)、褪黑素前干预组(M组)、对照组(C组). A组的大鼠腹腔注射6%左旋精氨酸(25 mL/kg体质量), 每次间隔1 h, 共3次, 诱发ANP, 且在诱发ANP前、后0.5 h腹腔注射生理盐水(20 mL/kg体质量)各1次; M组的大鼠诱发ANP的方法同A组, 但在诱发ANP前0.5 h腹腔注射0.25%褪黑素(20 mL/kg体质量)1次, 替代生理盐水; C组的大鼠腹腔注射相当于A组各次注射用量的等容积生理盐水. 每组术后6 h、12 h及24 h时点分批处置大鼠. 抽血测定Trx-1、白介素-6(IL-6)、谷胱甘肽(GSH)、总超氧化物歧化酶(T-SOD)、丙二醛(MDA)的含量; 取胰腺和肺组织做病理学检查.
结果: 大鼠胰腺组织的病理变化和病理学评分证实左旋精氨酸导致A组的ANP. 与A组比较, M组胰腺和肺组织的病理损伤显著减轻. 与C组比较, A组的大鼠血清的Trx-1的量在6 h、12 h时点显著降低, 24 h时点显著升高, 呈现出先降低后升高的趋势; 与A组比较, M组的大鼠血清的Trx-1的量在6 h、12 h时点显著增高, 他们表达的峰值前移. 与C组比较, A组的大鼠血清的IL-6、MDA水平显著增高, 血清的T-SOD活力、GSH的含量显著降低; 与A组比较, M组的大鼠血清的IL-6、MDA水平显著降低, 血清的T-SOD活力、GSH的含量显著增高.
结论: ANP可诱导Trx-1的表达; 而褪黑素前干预可进一步促进Trx-1的表达, 提高T-SOD活力和GSH水平, 降低IL-6、MDA水平, 故能显著减轻大鼠胰腺和肺组织的病理损伤, 发挥其对胰腺和肺组织的保护作用.
引文著录: 钟卫一, 唐国都, 梁志海, 黄文福, 梁金仙, 王珺平. 褪黑素对大鼠急性坏死性胰腺炎的干预作用及机制. 世界华人消化杂志 2009; 17(23): 2352-2356
Revised: July 22, 2009
Accepted: July 27, 2009
Published online: August 18, 2009
AIM: To investigate the relationship between the protective effects of melatonin pre-intervention for the pancreas and lungs and the expression of thioredoxin-1 (Trx-1) in rats with acute necrotizing pancreatitis (ANP).
METHODS: Seventy-two male Spraque-Dawley rats were randomly divided into three groups: normal control group, ANP group and melatonin treatment group. The ANP model rats were induced by giving rats three intraperitoneal injections of 6% L-arginine at a dose of 25 mL/kg body weight at an interval of 1 h. In the normal control group, normal rats were intraperitoneally injected with normal saline. Rats in the ANP group were intraperitoneally injected with normal saline one half hour before and after ANP induction, while rats in the melatonin treatment group were intraperitoneally injected with a single dose of 0.25% melatonin (20 mL/kg body weight) one half hour prior to ANP induction. Rats were sacrificed at 6, 12 and 24 h after the last L-arginine injection, respectively. The pancreas, lungs and blood samples were quickly taken. The pathological changes in the pancreas and lungs were observed and scored. The serum contents of Trx-1, IL-6, glutathione (GSH), total superoxide dismutase (T-SOD) and malondialdehyde (MDA) were measured.
RESULTS: Pathological analysis proved that L-arginine administration induced ANP successfully. Compared to the ANP group, pathological changes in the pancreas and lungs in the melatonin treatment group were significantly alleviated. Compared to the normal control group, serum Trx-1 contents in the ANP group were significantly decreased at 6- and 12-h time points but significantly increased at 24-h time point, exhibiting an initial decrease followed by a gradual increase. Serum Trx-1 contents in the melatonin treatment group at 6- and 12-h time points were significantly higher than those in the ANP group. Compared to the normal control group, serum IL-6 and MDA levels in the ANP group were significantly elevated, whereas serum T-SOD and GSH levels were significantly decreased. In contrast, serum IL-6 and MDA levels in the melatonin treatment group were significantly decreased while serum T-SOD and GSH levels were significantly increased when compared with the ANP group.
CONCLUSION: ANP can induce the expression of Trx-1. Melatonin pre-intervention is able to further promote the expression of Trx-1, increase serum Trx-1, SOD, and GSH levels and decrease serum IL-6 and MDA levels, thereby reducing pancreatic and pulmonary injury and exerting a protective effect for the pancreas and lungs in ANP rats.
- Citation: Zhong WY, Tang GD, Liang ZH, Huang WF, Liang JX, Wang JP. Mechanisms underlying the effects of melatonin intervention on acute necrotizing pancreatitis in rats. Shijie Huaren Xiaohua Zazhi 2009; 17(23): 2352-2356
- URL: https://www.wjgnet.com/1009-3079/full/v17/i23/2352.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v17.i23.2352
急性坏死性胰腺炎(acute necrotizing pancreatitis, ANP)是一种病死率较高的疾病. 研究揭示氧化应激、炎性细胞浸润在该疾病发生发展过程中发挥重要作用. 褪黑素是非酶类抗氧化物质, 是已知最强的自由基清除剂. 硫氧还蛋白是一种氧化还原蛋白, 具有抗氧化和抗炎作用. 本文通过建立大鼠ANP动物模型, 并给予外源性褪黑素前干预, 旨在观察褪黑素前干预对ANP胰腺和肺组织的保护作用及对硫氧还蛋白1(thioredoxin 1, Trx-1)表达的影响.
健康♂SD大鼠72只, 清洁级, 体质量200-250 g, 由广西医科大学实验动物中心提供; 左旋精氨酸和褪黑素购于Sigma公司; 大鼠Trx-1 ELISA试剂盒由Adlitteram诊断实验室提供; 大鼠白介素6(interleukin-6, IL-6)ELISA试剂盒购于晶美生物公司; 谷胱甘肽(glutathione, GSH)、SOD、MDA试剂盒购于南京建成生物工程研究所.
1.2.1 造模及分组: 所有大鼠造模前禁食12 h, 自由饮水. SD大鼠随机分成ANP组(A组)、褪黑素前干预组(M组)和对照组(C组), 每组各24只. A组的大鼠腹腔注射6%左旋精氨酸(25 mL/kg体质量), 每次间隔1 h, 共3次, 诱发ANP, 且在诱发ANP前、后半小时腹腔注射生理盐水(20 mL/kg体质量)各1次; M组的大鼠诱发ANP的方法同A组, 但在诱发ANP前半小时腹腔注射0.25%褪黑素(20 mL/kg体质量)1次, 替代生理盐水; C组的大鼠腹腔注射等容积生理盐水作为对照. 术后6 h、12 h及24 h每组每时点分别处死8只大鼠, 留取动脉血、胰腺和肺组织标本待测.
1.2.2 胰腺和肺组织病理学检查: 胰腺和肺组织常规固定、石蜡包埋、切片及HE染色. 光镜下观察大鼠胰腺和肺组织病理改变. 胰腺组织病理学评分参考Kusske et al[1]胰腺损伤的评分标准, 肺组织病理学评分参考Liu et al[2]肺损伤的评分标准.
1.2.3 Trx-1、IL-6的测定: 按ELISA试剂盒说明书操作检测.
1.2.4 GSH、总超氧化物歧化酶(total superoxide dismutase, T-SOD)、MDA的测定: 按测试盒的操作说明书进行.
统计学处理 应用SPSS13.0统计分析软件处理, 各组计量资料以mean±SD表示, 行单因素方差分析, P<0.05有统计学意义.
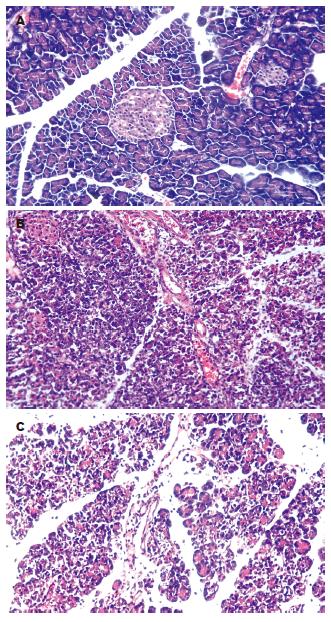
C组的大鼠胰腺肉眼观无变化; 镜下见胰腺腺泡结构完整, 腺小叶清晰, 偶见水肿或少量的炎性细胞浸润. A组胰腺外观灰白或暗紫色, 腹腔内有淡黄色或血性腹水, 可见部分腹腔脏器粘连、脂肪皂化; 镜下见胰腺腺泡水肿, 炎性细胞浸润, 组织坏死出血, 腺小叶结构消失, 偶见灶性或大片凝固性坏死; 病理变化并随时间延长而加重. M组的病变较A组显著减轻(图1, 表1).
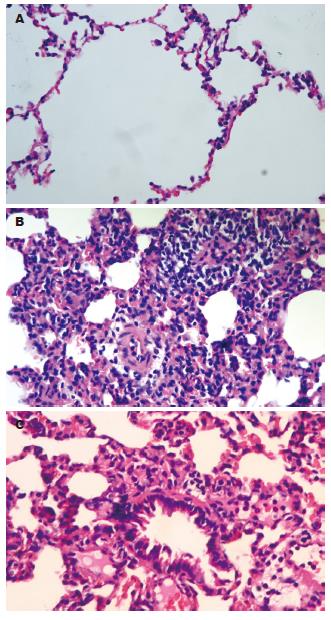
C组的大鼠镜下见肺组织结构完整, 偶见水肿或少量的炎性细胞浸润. A组的大鼠镜下可见肺间质和肺泡水肿, 炎性细胞浸润, 间质增宽, 毛细血管淤血, 间质和肺泡出血水肿, 甚至灶性或片状肺不张, 病理变化随时间延长而加重. M组的病变较A组显著减轻(图2, 表1).
与C组比较, A组的大鼠血清的Trx-1含量在6、12 h时值显著降低, 24 h时值显著升高, 呈现出前低后高的趋势; 与A组比较, M组血清的Trx-1含量在6、12 h时显著增高, 24 h时显著降低, 量的峰值前移. 与C组比较, A组的各时点和M组的6、12 h时大鼠血清的IL-6含量显著增高; 与A组比较, M组在6、12 h时血清的IL-6含量显著降低(表2).
与C组比较, A组的大鼠血清的T-SOD活力、GSH的含量显著降低, MDA的水平显著增高; M组的大鼠血清的T-SOD活力、GSH的含量无显著差异, 但MDA的水平12、24 h时点显著增高. 与A组比较, M组的大鼠血清表达的T-SOD活力、GSH的含量显著增高, 而MDA的含量在12、24 h时点显著降低(表3).
| 观察项目 | 分组 | 6 h | 12 h | 24 h |
| GSH(mg/L) | C组 | 2.998±0.741 | 3.157±0.732 | 3.497±0.913 |
| A组 | 1.341±0.592a | 1.869±0.657a | 1.980±0.657a | |
| M组 | 2.964±0.791c | 3.362±0.908c | 3.024±0.423c | |
| T-SOD(U/L) | C组 | 136.21±26.85 | 141.00±28.66 | 131.75±24.27 |
| A组 | 85.93±42.74a | 97.48±43.48a | 94.12±31.27a | |
| M组 | 165.50±37.18c | 155.75±44.10c | 130.75±36.21c | |
| MDA (μmol/L) | C组 | 1.681±0.261 | 1.846±0.597 | 1.865±0.566 |
| A组 | 2.308±0.522a | 3.989±1.078a | 4.959±1.141a | |
| M组 | 1.959±0.700 | 2.564±0.954ac | 3.455±0.949ac |
研究表明急性胰腺炎的加重与氧化应激相关[3]. 在急性胰腺炎早期阶段, 炎性细胞和腺泡细胞就产生大量的活性氧, 在细胞膜, 活性氧使多价不饱和脂肪酸过氧化导致膜的破坏和MDA(脂质过氧化反应的产物)生成[4]; 在细胞内, 活性氧破坏细胞结构导致胰酶的胞内运输紊乱和胰酶的过早激活而损伤腺泡细胞; 大量活性氧和破碎细胞泄漏的活化胰酶损伤毛细血管内皮, 毛细血管渗透性增加促成组织水肿[5]. 活性氧还可诱导IkB-α磷酸化, 激活核因子-kB(nuclear factor kappa B, NF-kB), 调节基因表达的炎症介质(TNF-α、IL-1β、IL-6和IL-8等), 扩增的炎症介质使局部的胰腺炎症扩展为全身炎症反应, 可进一步导致多器官功能障碍[6].
褪黑素是松果体分泌的主要激素, 具有多种生物学功能, 近年来, 研究的重点向其抗氧化作用倾斜, 越来越多的研究表明他能保护细胞核DNA、膜脂质、胞质蛋白等生物大分子免受氧化损伤[7], 能有效地清除多种自由基和过氧化氢[8]. 实验表明褪黑素能减轻多种物质诱导的多种组织的氧化应激反应. 如, 他能减轻内毒素血症诱导的幼鼠肠的氧化应激及细胞凋亡[9]; 弱化砷诱导的细胞氧化应激造成的肝肾损害[10]; 抵抗g射线照射引起的鼠的氧化应激和组织损伤[11]. 在雨蛙素诱导的大鼠ANP模型, 褪黑素能降低胰腺及肝脏组织的MDA的含量, 提高过氧化氢酶、谷胱甘肽过氧化物酶的含量, 从而减轻胰腺及肝脏组织的病理损伤[12]. 褪黑素前干预ANP能显著的减轻胰腺组织的脂质过氧化反应、减少髓过氧化酶及IL-6的含量[13].
Trx-1作为一种内生的抗氧化剂, 在组织的超氧化物歧化酶和谷胱甘肽过氧化物酶被耗尽后可能发挥关键性作用[14]. Trx-1在胞质通过直接清除活性氧发挥抗氧化作用, 在胞核通过抑制IkB-α的磷酸化, 遏制NF-kB的活化, 减少致炎细胞因子的表达, 发挥间接抗炎、抗氧化作用[14]. 给予外源性Trx-1可清除细胞外的活性氧, 还能穿透细胞膜, 进入胞质清除细胞内活性氧[15]. 在雨蛙素诱导急性胰腺炎动物模型, Trx-1的高表达可减轻急性胰腺炎氧化应激反应, 同时减轻TNF、IL-1等促炎细胞因子的激活, 从而减轻实验动物胰腺、肺、肝的损伤, 降低实验动物的死亡率[16].
本研究显示, 在左旋精氨酸诱导的非转基因大鼠的ANP模型, 血清Trx-1的含量呈现随时间延长先降低后升高的趋势, 在24 h时达峰值, 表明ANP可诱导Trx-1的表达, 体现了机体的自我保护机制. 而褪黑素前干预可显著地提高血清Trx-1含量、T-SOD活力和GSH水平, 降低IL-6、MDA水平, 且能显著减轻大鼠胰腺和肺组织的病理损伤, 这表明褪黑素通过激活抗氧化酶类的活性, 减少炎症介质的产生, 从而减轻全身炎症反应, 发挥对胰腺和肺等组织的保护作用.
急性坏死性胰腺炎的发病与氧化应激密切相关. 氧化应激时产生大量氧化物(主要是氧自由基)造成胰腺细胞的损伤, 这是急性坏死性胰腺炎发病的重要机制之一. 硫氧还蛋白和褪黑素都有抗氧化作用, 褪黑素干预对Trx-1表达造成何种影响, 至今尚无两者相互作用的研究报道.
姜慧卿, 教授, 河北医科大学第二医院消化科.
褪黑素是松果体分泌的主要激素, 具有多种生理功能. 近年来, 研究的重点向其抗氧化作用倾斜. 目前认为其抗氧化作用的机制主要包括两方面: 一方面, 他能以电子供体的形式直接中和大量的自由基、活性氧和一氧化氮; 另一方面, 通过激活若干抗氧化酶类的活性, 起间接的抗氧化作用.
Ohashi et al检测重症急性胰腺炎和轻型急性胰腺炎患者血清Trx-1的水平, 结果提示前者高于后者, 他们认为Trx-1是评价重症急性胰腺炎的严重性与氧化应激的一个可靠指标. 他们还发现转基因小鼠由于高表达Trx-1, 在诱导急性胰腺炎时氧化应激反应显著减轻.
本研究在大鼠ANP模型上首次探讨褪黑素干预对Trx-1表达的影响, 发现褪黑素干预可提高Trx-1的表达水平.
本研究选题尚可, 设计合理, 结果可信, 具有一定的学术价值.
编辑: 李军亮 电编:何基才
| 1. | Kusske AM, Rongione AJ, Ashley SW, McFadden DW, Reber HA. Interleukin-10 prevents death in lethal necrotizing pancreatitis in mice. Surgery. 1996;120:284-288; discussion 289. [PubMed] [DOI] |
| 2. | Liu D, Zeng BX, Shang Y. Decreased expression of peroxisome proliferator-activated receptor gamma in endotoxin-induced acute lung injury. Physiol Res. 2006;55:291-299. [PubMed] |
| 3. | Gómez-Cambronero LG, Sabater L, Pereda J, Cassinello N, Camps B, Viña J, Sastre J. Role of cytokines and oxidative stress in the pathophysiology of acute pancreatitis: therapeutical implications. Curr Drug Targets Inflamm Allergy. 2002;1:393-403. [PubMed] [DOI] |
| 4. | Dabrowski A, Gabryelewicz A, Wereszczyńska-Siemiatkowska U, Chyczewski L. Oxygen-derived free radicals in cerulein-induced acute pancreatitis. Scand J Gastroenterol. 1988;23:1245-1249. [PubMed] [DOI] |
| 5. | Dabrowski A, Konturek SJ, Konturek JW, Gabryelewicz A. Role of oxidative stress in the pathogenesis of caerulein-induced acute pancreatitis. Eur J Pharmacol. 1999;377:1-11. [PubMed] [DOI] |
| 6. | Wulczyn FG, Krappmann D, Scheidereit C. The NF-kappa B/Rel and I kappa B gene families: mediators of immune response and inflammation. J Mol Med. 1996;74:749-769. [PubMed] [DOI] |
| 7. | Reiter RJ, Tan DX, Allegra M. Melatonin: reducing molecular pathology and dysfunction due to free radicals and associated reactants. Neuro Endocrinol Lett. 2002;23 Suppl 1:3-8. [PubMed] |
| 8. | Poeggeler B, Saarela S, Reiter RJ, Tan DX, Chen LD, Manchester LC, Barlow-Walden LR. Melatonin--a highly potent endogenous radical scavenger and electron donor: new aspects of the oxidation chemistry of this indole accessed in vitro. Ann N Y Acad Sci. 1994;738:419-420. [PubMed] |
| 9. | Ozdemir D, Uysal N, Tugyan K, Gonenc S, Acikgoz O, Aksu I, Ozkan H. The effect of melatonin on endotoxemia-induced intestinal apoptosis and oxidative stress in infant rats. Intensive Care Med. 2007;33:511-516. [PubMed] [DOI] |
| 10. | Pal S, Chatterjee AK. Possible beneficial effects of melatonin supplementation on arsenic-induced oxidative stress in Wistar rats. Drug Chem Toxicol. 2006;29:423-433. [PubMed] [DOI] |
| 11. | El-Missiry MA, Fayed TA, El-Sawy MR, El-Sayed AA. Ameliorative effect of melatonin against gamma-irradiation-induced oxidative stress and tissue injury. Ecotoxicol Environ Saf. 2007;66:278-286. [PubMed] [DOI] |
| 12. | Eşrefoğlu M, Gül M, Ates B, Batçioğlu K, Selimoğlu MA. Antioxidative effect of melatonin, ascorbic acid and N-acetylcysteine on caerulein-induced pancreatitis and associated liver injury in rats. World J Gastroenterol. 2006;12:259-264. [PubMed] |
| 13. | Szabolcs A, Reiter RJ, Letoha T, Hegyi P, Papai G, Varga I, Jarmay K, Kaszaki J, Sari R, Rakonczay Z Jr, Lonovics J, Takacs T. Effect of melatonin on the severity of L-arginine-induced experimental acute pancreatitis in rats. World J Gastroenterol. 2006;12:251-258. [PubMed] |
| 14. | Hirota K, Murata M, Sachi Y, Nakamura H, Takeuchi J, Mori K, Yodoi J. Distinct roles of thioredoxin in the cytoplasm and in the nucleus. A two-step mechanism of redox regulation of transcription factor NF-kappaB. J Biol Chem. 1999;274:27891-27897. [PubMed] [DOI] |
| 15. | Kondo N, Ishii Y, Kwon YW, Tanito M, Horita H, Nishinaka Y, Nakamura H, Yodoi J. Redox-sensing release of human thioredoxin from T lymphocytes with negative feedback loops. J Immunol. 2004;172:442-448. [PubMed] |
| 16. | Ohashi S, Nishio A, Nakamura H, Kido M, Ueno S, Uza N, Inoue S, Kitamura H, Kiriya K, Asada M. Protective roles of redox-active protein thioredoxin-1 for severe acute pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2006;290:G772-G781. [PubMed] [DOI] |