修回日期: 2009-07-12
接受日期: 2009-07-20
在线出版日期: 2009-08-18
目的: 观察脂联素在小鼠急性肝损伤的表达及作用.
方法: 健康♂BALB/c小鼠随机分为3组. 刀豆蛋白A(concanavalin A, ConA)组8只: ConA 30 mg/kg 尾静脉注射. 脂联素组5只: 在静脉注射ConA前12 h和2 h, 分别应用脂联素3 mg/kg腹膜内注射. 对照组8只: 尾静脉注射生理盐水10 mL/kg. 注射ConA 8 h后处死小鼠, 检测脂联素及ALT血清水平、肝组织病理变化、肝组织脂联素蛋白及mRNA的表达.
结果: 脂联素血清水平在脂联素组与对照组无差异, 2组均高于ConA组(15.4±3.0 mg/L, 16.5±2.8 mg/L vs 11.8±2.1 mg/L, P<0.05或0.01); 肝组织脂联素蛋白表达在脂联素组强于对照组(5.39%±1.72% vs 1.82%±0.36%, P<0.05), 弱于ConA组(10.63±4.35%, P<0.05); 肝组织脂联素mRNA表达在脂联素组和ConA组均强于对照组(0.46±0.17, 0.51±0.21 vs 0.23±0.05, P<0.05或0.01); 血清ALT水平及肝组织炎症程度脂联素组高于对照组(192.50±45.87 U/L vs 44.71±21.29 U/L; 21.5±9.2 vs 8.4±4.3, 均P<0.05), 低于ConA组(616.00±171.50 U/L, 48.5±8.6, 均P<0.05).
结论: 在ConA诱导的急性肝损伤中, 肝组织脂联素蛋白及其mRNA的表达增强, 但血清脂联素水平降低. 脂联素对急性肝损伤小鼠起着抗炎作用.
引文著录: 尹洪竹, 邸海灵, 曹治宸, 刘英辉, 刘芳. 脂联素在小鼠急性肝损伤中的表达及作用. 世界华人消化杂志 2009; 17(23): 2346-2351
Revised: July 12, 2009
Accepted: July 20, 2009
Published online: August 18, 2009
AIM: To investigate the expression and role of adiponectin in murine acute liver injury.
METHODS: Healthy male BALB/c mice were randomly divided into three groups: concanavalin A (ConA) treatment group (n = 8; intravenously injected with ConA at 30 mg/kg), adiponectin treatment group (n = 5; intraperitoneally injected with adiponectin at 3 mg/kg at 12 and 3 hours before ConA injection) and normal control group (n = 8; intravenously injected with normal saline at 10 mL/kg). Mice were sacrificed eight hours after ConA treatment to detect serum adiponectin and ALT levels, observe hepatic pathological changes and determine the expression of adiponectin mRNA and protein in the liver.
RESULTS: Though no significant difference was observed in serum adiponectin levels between the adiponectin treatment group and the normal control group, serum adiponectin levels in these two groups were significantly higher than that in the ConA treatment group (15.4 ± 3.0 mg/L and 16.5 ± 2.8 mg/L vs 11.8 ± 2.1 mg/L, respectively; P < 0.05 or 0.01). The expression intensity of adiponectin protein in the liver in the adiponectin treatment group was higher than that in the normal control group (5.39% ± 1.72% vs 1.82% ± 0.36%, P < 0.05) but lower than that in the ConA treatment group (10.63% ± 4.35%, P < 0.05). The expression levels of adiponectin mRNA in the liver in the adiponectin treatment group and the ConA treatment group were significantly higher than that in the normal control group (0.46 ± 0.17 and 0.51 ± 0.21 vs 0.23 ± 0.05, respectively; P < 0.05 or P < 0.01). The serum ALT level and the degree of hepatitis in the adiponectin treatment group were higher than those in the normal control group (192.50 ± 45.87 U/L vs 44.71 ± 21.29 U/L and 21.5 ± 9.2 vs 8.4 ± 4.3, respectively; both P < 0.05), but lower than those in the ConA treatment group (616.00 ± 171.50 U/L and 48.5 ± 8.6, respectively; both P < 0.01).
CONCLUSION: The expression of adiponectin mRNA and protein in the liver is upregulated in ConA-mediated acute liver injury though serum adiponectin level is lowered. Adiponectin may have antiinflammatory effects in ConA-induced acute liver injury.
- Citation: Yin HZ, Di HL, Cao ZC, Liu YH, Liu F. Expression and role of adiponectin in murine acute liver injury. Shijie Huaren Xiaohua Zazhi 2009; 17(23): 2346-2351
- URL: https://www.wjgnet.com/1009-3079/full/v17/i23/2346.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v17.i23.2346
脂联素是由脂肪细胞产生和表达的脂肪因子. 多年来人们一直认为白色脂肪组织是脂联素产生的唯一场所. 然而近年来研究显示, 在正常生理情况下, 骨骼肌、肾脏、输卵管、卵巢、垂体、脾均可检测出脂联素蛋白[1-5], 肝脏也可表达脂联素, 且在许多疾病情况下, 脂联素的表达可发生变化. 如: 非酒精性脂肪性肝炎患者脂联素及其mRNA的表达显著低于单纯性脂肪肝患者[6]及健康对照者[7]; 小鼠注射CCl4后, 肝组织脂联素的表达增加[8]. 那么在刀豆蛋白A(concanavalin A, ConA)诱导的急性肝损伤中, 脂联素的表达是增强还是减弱, 脂联素在急性肝损伤中起什么作用, 本实验对此进行了观察.
健康♂BALB/c小鼠21只, 周龄4 wk, 体质量20±3 g, 购自河北医科大学动物实验中心(清洁级, 实验动物使用许可证号: SCXK(冀) 2003-1-003); 血清脂联素检测试剂盒由上海森雄科技实业有限公司提供; 小鼠脂联素mAb购于英国Abcam公司.
1.2.1 分组及给药: 小鼠用标准饲料喂养1 wk后, 随机分为3组. ConA组(n = 8): 尾静脉注射ConA 30 mg/kg. 对照组(n = 8): 尾静脉注射生理盐水10 mL/kg. 脂联素组(n = 5): 在静脉注射ConA前12 h和2 h, 分别应用脂联素3 mg/kg腹膜内注射(其余2组同时给予相当剂量的生理盐水腹膜内注射). 实验动物在注射ConA前12 h开始禁食.
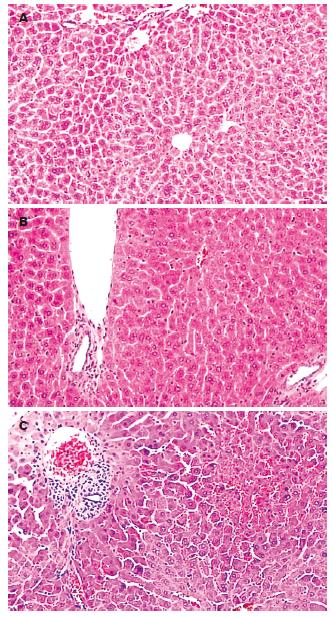
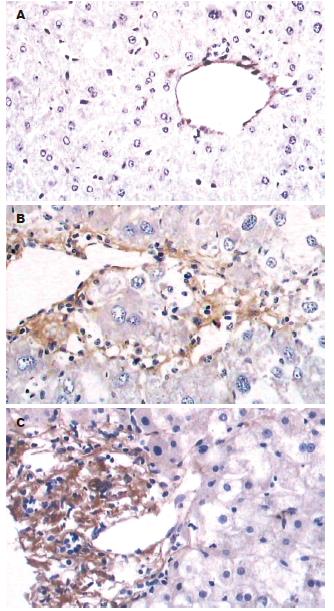
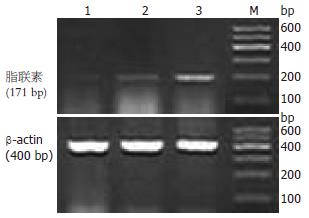
1.2.2 取材及指标检测: 注射ConA 8 h后股动脉放血处死小鼠, 留取血清及肝组织. 血清脂联素的检测采用酶联免疫吸附试验法; 肝组织脂联素检测采用免疫组织化学法; 脂联素mRNA采用逆转录-聚合酶链反应检测, 脂联素引物序列依据文献设计, 由上海生物工程公司合成: 上游: 5'-GTCAGTGGATCTGACGACACCAA-3', 下游: 5'-ATGCCTGCCATCCA ACCTG-3', 扩增产物为171 bp; ALT的检测采用速率法. 病理学检查采用常规HE染色, 观察肝组织病理变化(肝组织炎症和细胞病变的程度). 用病理指标评定肝损伤程度. 病理指数的确定[9]: 在高倍镜下每次取20个视野, 每一视野分3等份;"0"记整个视野无组织炎症及肝细胞病变;"1"记发生上述病变<1/3视野;"2"记发生上述病变1/3-2/3视野;"3"记发生上述病变>2/3视野; 20个视野得分相加即为肝细胞的病理指数PI值.
统计学处理 使用SPSS12.0统计软件处理实验数据, 所有数据以mean±SD表示, 样本均数比较采用秩和检验, P<0.05认为有显著性差异.
光镜下, 对照组小鼠肝细胞以中央静脉为中心呈放射状排列, 汇管区无炎症细胞浸润. ConA组可见肝内大量T淋巴细胞浸润, 在汇管区尤为明显, 肝窦内红细胞淤积, 中央静脉周围肝细胞水肿明显, 可见点、灶状坏死区. 脂联素组肝组织炎症细胞浸润明显减轻, 无明显淤血, 可见肝细胞轻度水肿(图1). 各组病理变化用病理指数评定(表1).
对照组、脂联素组、ConA组ALT水平逐渐升高, 统计学分析显示, 脂联素组血清ALT水平高于对照组, ConA组ALT水平高于脂联素组和对照组(表1).
对照组、脂联素组、ConA组血清脂联素水平逐渐下降, 统计学分析显示, 脂联素组与对照组比较无差异, 2组均高于ConA组(表2).
对照组小鼠肝组织脂联素蛋白表达阳性, 主要表达在中央静脉内皮细胞. ConA组和脂联素组肝组织脂联素蛋白表达增强, 主要表达于炎症细胞浸润区、门脉血管和部分肝窦内皮(图2). 以平均面密度表示蛋白表达量见表2.
本实验结果显示, 小鼠尾静脉注射ConA, 可引起肝脏炎症细胞浸润、肝窦淤血、肝细胞水肿及灶状坏死, 为典型肝炎病变. 如在ConA攻击前用脂联素干预, 可减轻ConA诱导的肝脏炎症细胞浸润, 减少肝窦淤血, 证明脂联素具有减轻ConA所致的小鼠急性肝损伤的作用. 其机制与脂联素促进巨噬细胞产生抗炎细胞因子白介素-10(interleukin-10, IL-10)、负向调节NK细胞的活性有关[10-12]. 最近研究还显示[13]: 脂联素对于肝再生也起着重要作用.
脂联素主要由脂肪组织表达和分泌, 但在适当的刺激下也可表达在脂肪细胞以外的其他细胞. 本实验显示, 正常小鼠肝组织即可有脂联素蛋白的表达, 在ConA诱导的急性肝损伤可增强脂联素蛋白在肝脏的表达, 这与Wolf et al报道一致[14]. 那么, 脂联素增强表达的机制是什么? Delaigle et al[15]应用脂多糖注射小鼠, 发现脂联素在骨骼肌的表达增强, 用炎症因子肿瘤细胞坏死因子-α(tumor necrosis factor-α, TNF-α)、干扰素-γ(interferon γ, INF-γ)、IL-6、IL-1干预体外培养的骨骼肌细胞, 发现TNF-α、INF-γ联合干预组脂联素水平明显升高, 且应用一氧化氮合成抑制剂则减少脂联素表达. 在ConA诱导的肝损伤中, 上述炎症因子和NO含量均增加[16], 推测在ConA诱导的急性炎症情况下, 肝组织脂联素表达增强与炎症细胞因子和NO的增高有关. 用IL-6孵育人肝癌HepG2细胞可观察到脂联素的诱导表达支持这一观点. 本文结果还显示: 脂联素在炎症细胞浸润区表达增强. 体外研究显示[14], 正常肝组织来源的巨噬细胞即可表达脂联素mRNA. 在ConA刺激下, 肝组织淋巴细胞浸润增多, 尤其在汇管区, 推测淋巴细胞表达脂联素增强, 脂联素通过与其受体结合而发挥生物学作用.
炎症情况下肝组织脂联素增强表达具有什么生理意义呢? 脂联素在多种细胞系均是TNF-α产生的对抗剂[17-19], 并可减轻TNF-α诱导的原代肝细胞死亡[20]. Maeda et al[21]报道脂联素缺乏小鼠脂肪组织显示高水平TNF-α mRNA和血浆浓度, 但如果将脂联素基因高效表达载体导入小鼠体内或补充外源性脂联素, 均可降低TNF-α血清水平[22-23]. 而且本文结果显示, 注射外源脂联素, 可降低ConA诱导的小鼠ALT水平. 因此, 在肝组织急性炎症情况下脂联素的增强表达可能是对抗促炎因子TNF-α而形成的负反馈弧以抗衡肝脏炎症. 研究还显示[24-25], 脂联素诱导人白细胞抗炎细胞因子IL-10和IL-1RA的产生, 抑制促炎因子IL-8的产生, 这些资料均提示脂联素有抑制炎症反应的作用.
尽管在急性炎症条件下, 肝组织和骨骼肌脂联素的表达增加, 但本研究结果显示, 注射ConA后小鼠血清脂联素水平是下降的. 考虑其原因与脂肪组织脂联素表达下降有关. TNF-α是ConA诱导肝损害的首要介质, TNF-α可在转录水平抑制3T3-L1脂肪细胞脂联素的表达并减少原代人脂肪细胞脂联素蛋白的表达和分泌[24]; Saijo et al[25]研究也发现, TNF-α, IL-1β和IFN-γ以剂量和时间依赖的方式抑制人脂肪细胞总脂联素的分泌; IL-6、IL-18也可抑制培养的3T3-L1脂肪细胞脂联素mRNA和蛋白的表达[26-27]. 应用可溶性TNF-α受体可使循环脂联素水平升高, 并减轻ConA诱导的肝损伤(ALT水平下降), 升高脂肪组织脂联素mRNA表达[28]. 说明促炎细胞因子抑制脂肪细胞脂联素的分泌和表达. 另外, NO也抑制3T3-L1脂肪细胞脂联素的表达[29-31]. 注射ConA抑制脂肪组织生成脂联素而导致循环脂联素水平的下降, 表明脂肪组织脂联素的分泌对循环脂联素的定量起着决定性的作用. 但ConA诱导肝损害不排除在肌肉或肝脏的局部以与脂肪组织相反的方式对脂联素水平进行调节.
总之, ConA诱导的小鼠急性肝损伤时, 肝组织脂联素表达增加, 而循环脂联素水平降低, 补充外源性脂联素可明显降低血清ALT水平, 对肝损伤呈现一种保护作用.
目前研究证实脂联素有一定的抗炎作用. 那么, 对病毒性肝炎脂联素是否也能起到抗炎作用? 刀豆蛋白A诱导的肝炎病理生理特点与人急性肝炎相似, 故本文观察了脂联素在ConA诱导的急性肝炎的表达及对炎症的作用, 旨在为病毒性肝炎的治疗提供新途径.
戴朝六, 教授, 中国医科大学第二临床学院(盛京医院)肝胆外科.
本文不仅观察了脂联素对ConA诱导的小鼠急性肝损伤的保护作用, 而且还观察了脂联素在急性肝损伤小鼠肝脏及血清中的表达变化, 并阐述其原因.
本研究表明脂联素对刀豆蛋白A(ConA)所致急性肝损伤的干预作用, 具有一定的临床应用价值及理论指导意义, 有很好的社会效益和经济前景.
本研究探讨了急性肝损伤时肝组织脂联素表达及其意义, 并分析了肝组织脂联素表达与血清脂联素水平之间产生剪刀差的原因, 内容有一定的科学性、创新性和可读性, 一定程度上反映了我国胃肠病学基础研究的水平.
编辑: 李军亮 电编:何基才
| 1. | Punyadeera C, Zorenc AH, Koopman R, McAinch AJ, Smit E, Manders R, Keizer HA, Cameron-Smith D, van Loon LJ. The effects of exercise and adipose tissue lipolysis on plasma adiponectin concentration and adiponectin receptor expression in human skeletal muscle. Eur J Endocrinol. 2005;152:427-436. [PubMed] [DOI] |
| 2. | Rovin BH, Song H, Hebert LA, Nadasdy T, Nadasdy G, Birmingham DJ, Yung Yu C, Nagaraja HN. Plasma, urine, and renal expression of adiponectin in human systemic lupus erythematosus. Kidney Int. 2005;68:1825-1833. [PubMed] [DOI] |
| 3. | Archanco M, Gómez-Ambrosi J, Tena-Sempere M, Frühbeck G, Burrell MA. Expression of leptin and adiponectin in the rat oviduct. J Histochem Cytochem. 2007;55:1027-1037. [PubMed] [DOI] |
| 4. | Chabrolle C, Tosca L, Dupont J. Regulation of adiponectin and its receptors in rat ovary by human chorionic gonadotrophin treatment and potential involvement of adiponectin in granulosa cell steroidogenesis. Reproduction. 2007;133:719-731. [PubMed] [DOI] |
| 5. | Maddineni S, Metzger S, Ocón O, Hendricks G 3rd, Ramachandran R. Adiponectin gene is expressed in multiple tissues in the chicken: food deprivation influences adiponectin messenger ribonucleic acid expression. Endocrinology. 2005;146:4250-4256. [PubMed] [DOI] |
| 6. | Kaser S, Moschen A, Cayon A, Kaser A, Crespo J, Pons-Romero F, Ebenbichler CF, Patsch JR, Tilg H. Adiponectin and its receptors in non-alcoholic steatohepatitis. Gut. 2005;54:117-121. [PubMed] [DOI] |
| 7. | Pagano C, Soardo G, Esposito W, Fallo F, Basan L, Donnini D, Federspil G, Sechi LA, Vettor R. Plasma adiponectin is decreased in nonalcoholic fatty liver disease. Eur J Endocrinol. 2005;152:113-118. [PubMed] [DOI] |
| 8. | Yoda-Murakami M, Taniguchi M, Takahashi K, Kawamata S, Saito K, Choi-Miura NH, Tomita M. Change in expression of GBP28/adiponectin in carbon tetrachloride-administrated mouse liver. Biochem Biophys Res Commun. 2001;285:372-377. [PubMed] [DOI] |
| 10. | Huang H, Park PH, McMullen MR, Nagy LE. Mechanisms for the anti-inflammatory effects of adiponectin in macrophages. J Gastroenterol Hepatol. 2008;23 Suppl 1:S50-S53. [PubMed] [DOI] |
| 11. | Park PH, McMullen MR, Huang H, Thakur V, Nagy LE. Short-term treatment of RAW264.7 macrophages with adiponectin increases tumor necrosis factor-alpha (TNF-alpha) expression via ERK1/2 activation and Egr-1 expression: role of TNF-alpha in adiponectin-stimulated interleukin-10 production. J Biol Chem. 2007;282:21695-21703. [PubMed] [DOI] |
| 12. | Kim KY, Kim JK, Han SH, Lim JS, Kim KI, Cho DH, Lee MS, Lee JH, Yoon DY, Yoon SR. Adiponectin is a negative regulator of NK cell cytotoxicity. J Immunol. 2006;176:5958-5964. [PubMed] |
| 13. | Ezaki H, Yoshida Y, Saji Y, Takemura T, Fukushima J, Matsumoto H, Kamada Y, Wada A, Igura T, Kihara S. Delayed liver regeneration after partial hepatectomy in adiponectin knockout mice. Biochem Biophys Res Commun. 2009;378:68-72. [PubMed] [DOI] |
| 14. | Wolf AM, Wolf D, Avila MA, Moschen AR, Berasain C, Enrich B, Rumpold H, Tilg H. Up-regulation of the anti-inflammatory adipokine adiponectin in acute liver failure in mice. J Hepatol. 2006;44:537-543. [PubMed] [DOI] |
| 15. | Delaigle AM, Jonas JC, Bauche IB, Cornu O, Brichard SM. Induction of adiponectin in skeletal muscle by inflammatory cytokines: in vivo and in vitro studies. Endocrinology. 2004;145:5589-5597. [PubMed] [DOI] |
| 17. | Masaki T, Chiba S, Tatsukawa H, Yasuda T, Noguchi H, Seike M, Yoshimatsu H. Adiponectin protects LPS-induced liver injury through modulation of TNF-alpha in KK-Ay obese mice. Hepatology. 2004;40:177-184. [PubMed] [DOI] |
| 18. | Yokota T, Oritani K, Takahashi I, Ishikawa J, Matsuyama A, Ouchi N, Kihara S, Funahashi T, Tenner AJ, Tomiyama Y. Adiponectin, a new member of the family of soluble defense collagens, negatively regulates the growth of myelomonocytic progenitors and the functions of macrophages. Blood. 2000;96:1723-1732. [PubMed] |
| 19. | Park PH, Huang H, McMullen MR, Mandal P, Sun L, Nagy LE. Suppression of lipopolysaccharide-stimulated tumor necrosis factor-alpha production by adiponectin is mediated by transcriptional and post-transcriptional mechanisms. J Biol Chem. 2008;283:26850-26858. [PubMed] [DOI] |
| 20. | Sennello JA, Fayad R, Morris AM, Eckel RH, Asilmaz E, Montez J, Friedman JM, Dinarello CA, Fantuzzi G. Regulation of T cell-mediated hepatic inflammation by adiponectin and leptin. Endocrinology. 2005;146:2157-2164. [PubMed] [DOI] |
| 21. | Maeda N, Shimomura I, Kishida K, Nishizawa H, Matsuda M, Nagaretani H, Furuyama N, Kondo H, Takahashi M, Arita Y. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med. 2002;8:731-737. [PubMed] [DOI] |
| 23. | Xu A, Wang Y, Keshaw H, Xu LY, Lam KS, Cooper GJ. The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. J Clin Invest. 2003;112:91-100. [PubMed] |
| 24. | Wolf AM, Wolf D, Rumpold H, Enrich B, Tilg H. Adiponectin induces the anti-inflammatory cytokines IL-10 and IL-1RA in human leukocytes. Biochem Biophys Res Commun. 2004;323:630-635. [PubMed] [DOI] |
| 25. | Saijo S, Nagata K, Nakano Y, Tobe T, Kobayashi Y. Inhibition by adiponectin of IL-8 production by human macrophages upon coculturing with late apoptotic cells. Biochem Biophys Res Commun. 2005;334:1180-1183. [PubMed] [DOI] |
| 26. | Bruun JM, Lihn AS, Verdich C, Pedersen SB, Toubro S, Astrup A, Richelsen B. Regulation of adiponectin by adipose tissue-derived cytokines: in vivo and in vitro investigations in humans. Am J Physiol Endocrinol Metab. 2003;285:E527-E533. [PubMed] |
| 27. | Simons PJ, van den Pangaart PS, Aerts JM, Boon L. Pro-inflammatory delipidizing cytokines reduce adiponectin secretion from human adipocytes without affecting adiponectin oligomerization. J Endocrinol. 2007;192:289-299. [PubMed] [DOI] |
| 28. | Fasshauer M, Kralisch S, Klier M, Lossner U, Bluher M, Klein J, Paschke R. Adiponectin gene expression and secretion is inhibited by interleukin-6 in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2003;301:1045-1050. [PubMed] [DOI] |
| 29. | Chandrasekar B, Patel DN, Mummidi S, Kim JW, Clark RA, Valente AJ. Interleukin-18 suppresses adiponectin expression in 3T3-L1 adipocytes via a novel signal transduction pathway involving ERK1/2-dependent NFATc4 phosphorylation. J Biol Chem. 2008;283:4200-4209. [PubMed] [DOI] |
| 30. | Morris AM, Sennello JA, Fayad RA, Eckel RH, Dinarello CA, Fantuzzi G. T cell-mediated hepatic inflammation modulates adiponectin levels in mice: role of tumor necrosis factor alpha. Metabolism. 2006;55:555-559. [PubMed] [DOI] |
| 31. | Nozaki M, Fukuhara A, Segawa K, Okuno Y, Abe M, Hosogai N, Matsuda M, Komuro R, Shimomura I. Nitric oxide dysregulates adipocytokine expression in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2007;364:33-39. [PubMed] [DOI] |