修回日期: 2009-06-12
接受日期: 2009-06-15
在线出版日期: 2009-07-18
目的: 了解早期肠内谷氨酰胺补给对烫伤后肠黏膜屏障功能的保护作用, 并探讨其可能的作用机制.
方法: 选取健康SD大鼠随机分为标准肠内营养组(EN组)和谷氨酰胺补给组(EN+Gln组), 每组32只, 制成烫伤动物模型(30% TBSA Ⅲ度烫伤), 伤后早期分别给予标准肠内营养制剂(能全力)和标准肠内营养制剂+谷氨酰胺, 于第1、4、7、10天观察血清D-乳酸, Western blot半定量分析紧密连接结构蛋白Occludin和RT-PCR分析ZO-1 mRNA表达的变化.
结果: 烫伤后血清D-乳酸水平升高, EN组在观察时间内血清D-乳酸水平没有恢复至伤前水平, EN+Gln组在伤后4 d恢复(4.5±0.8 mg/L vs 3.8±0.6 mg/L); Western blot半定量分析显示EN+Gln组伤后Occludin表达先下降后上升, 在4、7 d时明显高于EN组(1.18±0.14 vs 0.79±0.09, 1.59±0.16 vs 1.12±0.13, 均P<0.05); 烧伤后1 d时, 2组ZO-1 mRNA表达与伤前比较均明显下降(0.71±0.19, 0.76±0.17 vs 1.00, 均P<0.05), 4 d时EN+Gln组与EN组比较差异有显著的统计学意义(1.17±0.16 vs 0.76±0.15, P<0.05), 其余时间点各组间差异均无统计学意义.
结论: 早期肠内谷氨酰胺补给支持能快速逆转烧伤后TJ结构蛋白Occludin、ZO-1表达的下降, 从而改善肠黏膜的屏障功能.
引文著录: 邓志云, 郭光华, 杨毅. 早期肠内谷氨酰胺补给对烫伤大鼠肠黏膜细胞紧密连接的影响. 世界华人消化杂志 2009; 17(20): 2031-2036
Revised: June 12, 2009
Accepted: June 15, 2009
Published online: July 18, 2009
AIM: To investigate the protective effects of early enteral glutamine supplementation on the barrier function of intestinal mucosa in scalded rats and explore the mechanism underlying such protective effects.
METHODS: Healthy adult SD rats were subjected to a 30% TBSA third-degree scald injury to develop a rat model of scald injury. Scalded rats were then randomly divided into EN and EN plus Gln group, respectively. Rats in the EN group were fed standard enteral nutrition (Nutrison Multi Fibre) while those in the EN plus Gln group were fed standard enteral nutrition plus Gln. On days 1, 4, 7 and 10 after scald induction, plasma D-lactic acid levels were analyzed by ultraviolet spectrophotometry, the expression of Occludin protein was detected by Western blot, and the expression of ZO-1 mRNA was determined by RT-PCR.
RESULTS: Serum D-lactic acid level was elevated in response to scald induction. The levels of D-lactic acid in the serum of rats in the EN group did not return to normal within the period of observation of this study, whereas those in the EN plus Gln group returned to normal on day 4 after scald induction (4.5 ± 0.8 mg/L vs 3.8 ± 0.6 mg/L). Semiquantitative Western blot analysis showed that the expression of Occludin protein in intestinal mucosa of rats in the EN plus Gln group showed an initial rise, followed by a decline. On days 4 and 7, the expression level of Occludin protein in intestinal mucosa of rats in the EN plus Gln group were significantly higher than those in the EN group (1.18 ± 0.14 vs 0.79 ± 0.09 and 1.59 ± 0.16 vs 1.12 ± 0.13, respectively; both P < 0.05). In both groups, the expression levels of ZO-1 mRNA on day 1 significantly declined when compared with pre-induction values (0.71 ± 0.19 and 0.76 ± 0.17 vs 1.00, both P < 0.05). On day 4, a significant difference was noted in the expression levels of ZO-1 mRNA between the two groups (1.17 ± 0.16 vs 0.76 ± 0.15; P < 0.05) though no significant differences were found at other time points.
CONCLUSION: Early enteral glutamine supplementation is superior to standard enteral nutrition in promoting the expression of Occludin protein and ZO-1 mRNA in intestinal mucosa and improving the barrier function of intestinal mucosa.
- Citation: Deng ZY, Guo GH, Yang Y. Effects of early enteral glutamine supplementation on tight junctions in intestinal mucosa of scalded rats. Shijie Huaren Xiaohua Zazhi 2009; 17(20): 2031-2036
- URL: https://www.wjgnet.com/1009-3079/full/v17/i20/2031.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v17.i20.2031
在严重烧伤、感染、休克等应激条件下, 因肠道缺血缺氧、炎性因子的瀑式释放、营养底物的缺乏等变化, 可引起上皮细胞间紧密连接通道的异常开放, 最终导致黏膜屏障功能损害. 近年来研究亦证实肠黏膜屏障功能下降和由此所致的肠源性细菌、内毒素移位、全身感染、多器官功能衰竭等并发症密切相关, 因此改善烧伤后肠黏膜屏障功能, 减少肠源性细菌、内毒素移位对于烧伤患者的临床和预后具有较大的现实意义[1-5]. 紧密连接(tight junction, TJ)是相邻上皮细胞间的连接方式细胞间最重要的连接方式, 控制着离子及小分子可溶性物质通过, 排斥毒性大分子及微生物侵入[6-9]. 近来研究发现谷氨酰胺(Gln)等能促进肠黏膜细胞的增生、防止肠黏膜萎缩及有利于维持肠黏膜屏障功能[10-13]. 为此我们设计了本实验, 对烫伤大鼠伤后早期肠内补给含谷氨酰胺的肠内营养制剂, 观察其对紧密连接主要构成蛋白Occludin和ZO-1的影响并探讨其可能的机制.
成年健康SD大鼠72只, 体质量220-250 g, 雌雄兼用, 由南昌大学医学院医学实验动物科学部提供(动物合格证: 医动字021-9602). 雌雄分笼, 适应性喂养1 wk, 自由摄水饮食. 主要试剂Occluding Rabbit mAb购自Santa Crutz公司; 以及相应的试剂购自Promega公司, PCR引物由上海生物技术服务公司合成.
1.2.1 大鼠烫伤模型的制作: SD大鼠随机分为2组, 即肠内营养组(EN组)和肠内营养加谷氨酰胺补给组(EN+Gln组), 2组所有大鼠均制成烫伤模型, 每组32只. 另取8只作为伤前对照, 制成假烫模型, 所得数据作为伤前参考值. 大鼠烫伤前禁食12 h, 10 g/L戊巴比妥钠(40 mg/kg)腹腔麻醉, 电推去毛, 称其质量后采用本实验中心自制蒸汽烫伤控制器, 设定压力为0.03 mPa, 温度108℃, 持续8 s, 造成总体表面积(TBSA)为30%的Ⅲ度烫伤. 伤后立即ip平衡液50 mL/kg抗休克. 将大鼠放入限制笼内饲养, 实验过程中室温保持在25℃左右, 相对湿度约40%左右, 创面外用碘伏消毒, 实验室每天紫外线消毒. 于伤后1, 4, 7和10 d检测观察指标, 在每个时间点每组大鼠均为8只.
1.2.2 营养液的配制与供给: 2组给予同等热量的肠内营养物. EN组给予整蛋白型肠内营养剂能全力(nutrison multi fibre), EN+Gln组在此基础上添加Gln(重庆药友制药有限公司惠赠)0.3 g/(d•kg); 2组在层流台上将各种营养成分混匀后分装, 4℃保存, 用时复温到37℃. 伤后4 h开始灌喂营养液, 按每天731.5 kJ/kg的标准供给[14], 每天计划量分3-5次喂完, 不限饮水.
1.2.3 检测指标与方法: (1)血清D-乳酸测定: 门静脉血离心后分离血清, -80℃保存, 用酶联紫外分光光度法测定血清D-乳酸水平. (2)Western blot法检测肠黏膜Occludin蛋白量: 载玻片刮取大鼠回肠黏膜层, 称质量后加入去污剂裂解缓冲液, 匀浆器匀浆, 冰上裂解30 min; 将已裂解的组织转移入1.5 mL EP管中, 4℃, 10 000 g离心10 min, 取上清; 取出上清液约100 μL, Folin-酚法进行蛋白质的定量及标化; SDS-聚丙烯酰胺凝胶电泳, 免疫学检测Occludin蛋白条带进行半定量分析; 具体步骤按参考文献[15]. (3)肠黏膜细胞ZO-1 mRNA检测: 每例标本取回肠黏膜组织约20 mg提取总RNA, Beckman DH7400分光光度计行质量、浓度检测与标化后, RNA样品逆转录、聚合酶链式反应(PCR)扩增. 引物序列见表1; 反应条件为: 94℃预变性3 min, 然后进行PCR反应35次循环: 94℃变性45 s, 47.5℃退火130 s, 72℃延伸60 s, 最后行72℃延伸5 min. 取扩增产物15 μL上样, 15 g/L琼脂糖凝胶电泳, 溴化乙啶染色显示后, 在透射紫外光分析仪下摄影, 并用激光光密度图像扫描仪扫描. 定量计算方法为: 待测基因吸光度值/内参照吸光度值, 再除以假烫组相对应样本的此2条带综合灰度之比.
| 分组 | Forward Primer (5'-3') | Reverse Primer (3'-5') | 大小(bp) |
| β-actin | CACCCTGTGCTGCTCACCGAGGCC | CCACACAGATGACTTGCGCTCAGG | 690 |
| ZO-1 | AATGCTGCTTTATTGGG | AGTGGTTGGTGGTCTTCT | 229 |
统计学处理 所有数据均用SPSS10.0统计软件进行分析, 用mean±SD进行统计描述, 同种处理不同时间间比较及同一时间内不同处理组间比较均采用单因素方差分析方法. 结果以P<0.05为差异显著, P<0.01为差异非常显著.
2组烫伤动物在1d时血清D-乳酸浓度较伤前明显升高(P<0.01), 在4、7及10 d时EN组与伤前比较D-乳酸浓度均显著升高(P<0.05); 在4、7及10 d时EN+Gln组与伤前比较差异无显著性意义(P>0.05). 在4、7 d时EN+Gln组血清D-乳酸浓度与EN组比较明显降低(P<0.05); 在其他时间点各组间比较差异均不明显(P>0.05). 这说明早期肠内谷氨酰胺补给能改善烫伤后肠黏膜屏障功能的损害(表2).
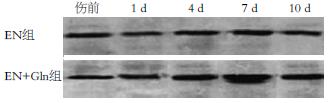
Western blot分析显示, 烫伤后1 d时2组动物Occludin的表达水平降低(与伤前比较P<0.05); EN组在伤后4 d回升, 7及10 d时与伤前比较差异无显著性意义(P>0.05); EN+Gln组在7 d时明显高于伤前(P<0.01), 4、10 d时与伤前比较差异无显著性意义(P>0.05, 图1). 组间比较在4、7 d时EN+Gln组与EN组比较差异均显示显著的统计学意义(P<0.01或0.05), 在其余时间点各组间比较差异均不明显(P>0.05); 说明伤后Gln补给能上调Occludin蛋白的表达(表3).
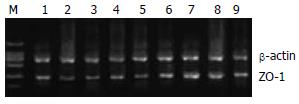
ZO-1 mRNA的RT-PCR分析显示, 2组动物烫伤后1 d时ZO-1 mRNA表达明显下降(与伤前比较P<0.05); EN组伤后4 d时仍低于伤前(P<0.05), 7、10 d时与伤前比较差异无显著的统计学意义(P>0.05); EN+Gln组在4、10 d时与伤前无差异(P>0.05). 提示烫伤后早期ZO-1 mRNA表达受到抑制, EN支持能逐渐逆转ZO-1 mRNA表达被抑制状态并恢复正常. 组间比较在4、7 d时EN+Gln组明显高于EN组(P<0.05); 说明相对于EN组肠内营养物, 早期Gln补给支持能在更快地促进ZO-1 mRNA表达的恢复, 从而改善TJ的通透性(表4, 图2-3).
紧密连接是肠腔相邻上皮细胞间最重要的连接方式, 他由一些特殊的蛋白组成(Occludin, Claudin, ZO-1、肌动蛋白等), 以上成分的复合结构形成防止微生物、微粒和大分子侵入的机械屏障[16-17]. 许多病理状态如烧伤、感染、休克等情况下, 引起细胞间紧密连接功能改变,细胞孔隙(窗孔)明显扩大, 导致大分子物质及毒素、细菌移位, 导致肠黏膜的通透性增高[18-21]. 在实验中我们利用Western blot分析了Occludin在各组之间的表达, 同时检测了ZO-1 mRNA的变化, 结果显示1 d时各组Occludin表达明显下降, 说明烧伤能引起紧密连接的破坏. Simonovic et al[22]在实验中证实在应激、感染等情况下, 由于钙耗竭等因素能导致上皮细胞间紧密连接的重要成分Occludin去磷酸化而致TJ的破坏. 谷氨酰胺补给组在4、7 d时Occludin表达明显高于EN组; 这说明Gln补给能有效地促进Occludin的表达, 下调上皮细胞间通透性. Occludin为一完整的Ⅱ型跨膜蛋白, 相对分子质量约65 kDa, 含4个跨膜结构, 位于紧密连接线上, 在维持和调节紧密连接屏障功能中具有重要作用[23-24]. Li et al[25]利用Caco-2细胞观察Gln对其紧密连接蛋白的影响, 发现Gln能促进Occludin的表达并呈剂量依赖性. 在CaCo-2细胞的体外培养体系中发现过氧化氢能快速诱导细胞紧密连接蛋白Occludin和ZO-1的Tyr残基磷酸化, 从而使紧密连接分解, 而EGF能完全阻止Tyr位点的磷酸化[26-28].
进一步的研究发现ERK的抑制剂能阻断EGF的效果; 在我们以前的研究中发现烧伤后早期肠内补给Gln能促进p-ERK1/2的表达[29]; 说明ERK通路的激活能维护细胞间的紧密连接的形成[30], 在他们的实验中发现p-ERK主要集中于上皮细胞的侧膜且与Occludin位置重叠, 随着EGF的给予, 二者定域也相应扩大, 利用GST-Occludin-C和重组ERK配对测定证实p-ERK1/2直接和Occludin的C末端相互作用, 阻止Occludin苏氨酸残基的去磷酸化, 引起TJ蛋白及其相关信号分子的磷酸化, 从而上调上皮细胞间TJ的完整性及其屏障功能.
ZO-1是膜相关鸟苷酸激酶家族成员之一, 与细胞质内的Occludin和Claudin相互作用, 同时也参与蛋白与蛋白相互作用的信号转导[31-32]. ZO-1能够与胞质内的其他蛋白如Occludins蛋白的C末端连接[33], 而ZO-1的C末端则可结合肌动蛋白和应激纤维, 从而将Occludin和肌动蛋白骨架系统连接在一起构成稳定的连接系统. 在Yang et al[34]的实验中利用发现ERK1/2的活化能促进肠黏膜ZO-1的表达, 用U0126阻断ERK1/2的活化, ZO-1和Occludin表达明显减少, 肠黏膜通透性升高. 在我们的实验中发现肠内Gln补给支持也能促进ZO-1 mRNA的表达. 结合TJ主要结构蛋白Occludin和p-ERK1/2的表达的变化[29], 可以确定早期肠内Gln补给对细胞间紧密连接起重要的正向调控作用.
为了评价不同营养内容对肠黏膜屏障功能影响的结果, 我们选择D-乳酸作为黏膜屏障通透性的标志物. 在正常情况下,哺乳动物组织既不产生D-乳酸, 也不代谢D-乳酸. 但在肠道应激缺血、创伤、感染等情况下, 肠道细菌产生的大量D-乳酸可通过受损的肠黏膜, 进入血液循环, 引起血清D-乳酸水平升高. 故检测血清D-乳酸水平可及时反映肠黏膜上皮细胞通透性及肠屏障功能的损害程度[35]. 本实验中各组烫伤动物在1 d时血清D-乳酸浓度较伤前均明显升高, 说明烫伤能明显引起肠黏膜通透性升高; 肠屏障功能处于损伤状态. 在4、7 d时EN+Gln组D-乳酸浓度明显低于EN组, 说明肠内Gln补给支持能快速、有效地增强肠黏膜屏障功能, 减少血清D-乳酸进入血液循环.
总之, 烫伤能引起TJ结构蛋白Occludin、ZO-1表达的减弱, 从而引起肠黏膜通透性升高, 肠黏膜屏障功能的损害. 肠内Gln补给支持能快速逆转烧伤后TJ结构蛋白Occludin、ZO-1表达下降, 从而上调肠黏膜的屏障功能. 这也是Gln改善烧伤后肠道功能的主要机制之一.
烧(创)伤后肠道应激性变化引起肠黏膜屏障功能损害, 细菌、内毒素移位乃至启动SIRS的发生, 并通过一系列级联反应可最终导致MODS; 烧伤后如何有效地促进肠黏膜修复, 维护肠黏膜屏障功能, 减少肠内细菌、内毒素移位并实行有效的营养支持成为烧伤治疗中的一个重要环节.
彭曦, 副研究员, 重庆市西南医院烧伤研究所
肠道是烧伤后高代谢反应的中心器官之一, 胃肠道的缺血缺氧性损害、营养底物的缺乏是导致黏膜机械屏障损害的主要原因. 围绕伤后黏膜屏障功能的维护开展了众多的研究; 亟待解决的问题是选择何种安全、有效的方法及干预的途径、时机.
Gln是21世纪的"明星营养素", 众多的研究报道了Gln提高机体免疫力、降低外科手术后感染并发症发生率、改善危重患者脓毒症及MODS发生率、缩短住院时间等方面的优势, 至于补给Gln对伤后紧密连接结构蛋白的影响目前尚未见报道.
肠内Gln补给支持能快速逆转烧伤后TJ结构蛋白Occludin、ZO-1表达下降, 从而上调肠黏膜的屏障功能.
本研究选题尚可, 设计合理, 结果可靠, 但内容稍显简单.
编辑: 李军亮 电编:吴鹏朕
| 1. | Wilmore DW. Nutrition and metabolic support in the 21st century. JPEN J Parenter Enteral Nutr. 2000;24:1-4. [PubMed] [DOI] |
| 2. | Briassoulis G, Filippou O, Hatzi E, Papassotiriou I, Hatzis T. Early enteral administration of immunonutrition in critically ill children: results of a blinded randomized controlled clinical trial. Nutrition. 2005;21:799-807. [PubMed] [DOI] |
| 4. | Wilson MD, Dziewulski P. Severe gastrointestinal haemorrhage and ischaemic necrosis of the small bowel in a child with 70% full-thickness burns: a case report. Burns. 2001;27:763-766. [PubMed] [DOI] |
| 5. | Xu GF, Lu Z, Gao J, Li ZS, Gong YF. Effect of ecoimmunonutrition supports on maintenance of integrity of intestinal mucosal barrier in severe acute pancreatitis in dogs. Chin Med J (Engl). 2006;119:656-661. [PubMed] |
| 6. | Cereijido M, Contreras RG, Flores-Bentez D, Flores-Maldonado C, Larre I, Ruiz A, Shoshani L. New diseases derived or associated with the tight junction. Arch Med Res. 2007;38:465-478. [PubMed] [DOI] |
| 7. | Gonzalez-Mariscal L, Betanzos A, Nava P, Jaramillo BE. Tight junction proteins. Prog Biophys Mol Biol. 2003;81:1-44. [PubMed] [DOI] |
| 8. | Matsuda M, Kubo A, Furuse M, Tsukita S. A peculiar internalization of claudins, tight junction-specific adhesion molecules, during the intercellular movement of epithelial cells. J Cell Sci. 2004;117:1247-1257. [PubMed] [DOI] |
| 10. | Coëffier M, Marion R, Ducrotté P, Déchelotte P. Modulating effect of glutamine on IL-1beta-induced cytokine production by human gut. Clin Nutr. 2003;22:407-413. [PubMed] [DOI] |
| 12. | Singleton KD, Beckey VE, Wischmeyer PE. Glutamine prevents activation of NF-kappaB and stress kinase pathways, attenuates inflammatory cytokine release, and prevents acute respiratory distress syndrome (ARDS) following sepsis. Shock. 2005;24:583-589. [PubMed] [DOI] |
| 15. | Mazzon E, Sturniolo GC, Puzzolo D, Frisina N, Fries W. Effect of stress on the paracellular barrier in the rat ileum. Gut. 2002;51:507-513. [PubMed] [DOI] |
| 16. | Matsuda M, Kubo A, Furuse M, Tsukita S. A peculiar internalization of claudins, tight junction-specific adhesion molecules, during the intercellular movement of epithelial cells. J Cell Sci. 2004;117:1247-1257. [PubMed] [DOI] |
| 17. | Hossain Z, Hirata T. Molecular mechanism of intestinal permeability: interaction at tight junctions. Mol Biosyst. 2008;4:1181-1185. [PubMed] [DOI] |
| 18. | Farmer DG, Venick RS. Morbidity and mortality associated with chronic intestinal failure. Transplantation. 2008;85:1385-1386. [PubMed] [DOI] |
| 19. | Vega D, Badami CD, Caputo FJ, Watkins AC, Lu Q, Xu da Z, Berezina TL, Zaets SB, Feketeova E, Deitch EA. The influence of the type of resuscitation fluid on gut injury and distant organ injury in a rat model of trauma/hemorrhagic shock. J Trauma. 2008;65:409-414; discussion 414-415. [PubMed] [DOI] |
| 20. | Zhang F, Tong L, Qiao H, Dong X, Qiao G, Jiang H, Sun X. Taurine attenuates multiple organ injury induced by intestinal ischemia reperfusion in rats. J Surg Res. 2008;149:101-109. [PubMed] [DOI] |
| 21. | Beale RJ, Sherry T, Lei K, Campbell-Stephen L, McCook J, Smith J, Venetz W, Alteheld B, Stehle P, Schneider H. Early enteral supplementation with key pharmaconutrients improves Sequential Organ Failure Assessment score in critically ill patients with sepsis: outcome of a randomized, controlled, double-blind trial. Crit Care Med. 2008;36:131-144. [PubMed] [DOI] |
| 22. | Simonovic I, Rosenberg J, Koutsouris A, Hecht G. Enteropathogenic Escherichia coli dephosphorylates and dissociates occludin from intestinal epithelial tight junctions. Cell Microbiol. 2000;2:305-315. [PubMed] [DOI] |
| 23. | Hirase T, Staddon JM, Saitou M, Ando-Akatsuka Y, Itoh M, Furuse M, Fujimoto K, Tsukita S, Rubin LL. Occludin as a possible determinant of tight junction permeability in endothelial cells. J Cell Sci. 1997;110:1603-1613. [PubMed] |
| 24. | Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993;123:1777-1788. [PubMed] [DOI] |
| 25. | Li N, Lewis P, Samuelson D, Liboni K, Neu J. Glutamine regulates Caco-2 cell tight junction proteins. Am J Physiol Gastrointest Liver Physiol. 2004;287:G726-G733. [PubMed] [DOI] |
| 26. | Sheth P, Seth A, Thangavel M, Basuroy S, Rao RK. Epidermal growth factor prevents acetaldehyde-induced paracellular permeability in Caco-2 cell monolayer. Alcohol Clin Exp Res. 2004;28:797-804. [PubMed] [DOI] |
| 27. | Atkinson KJ, Rao RK. Role of protein tyrosine phosphorylation in acetaldehyde-induced disruption of epithelial tight junctions. Am J Physiol Gastrointest Liver Physiol. 2001;280:G1280-G1288. [PubMed] |
| 28. | Kale G, Naren AP, Sheth P, Rao RK. Tyrosine phosphorylation of occludin attenuates its interactions with ZO-1, ZO-2, and ZO-3. Biochem Biophys Res Commun. 2003;302:324-329. [PubMed] [DOI] |
| 30. | Basuroy S, Seth A, Elias B, Naren AP, Rao R. MAPK interacts with occludin and mediates EGF-induced prevention of tight junction disruption by hydrogen peroxide. Biochem J. 2006;393:69-77. [PubMed] [DOI] |
| 31. | Baltes S, Nau H, Lampen A. All-trans retinoic acid enhances differentiation and influences permeability of intestinal Caco-2 cells under serum-free conditions. Dev Growth Differ. 2004;46:503-514. [PubMed] [DOI] |
| 32. | Weiler F, Marbe T, Scheppach W, Schauber J. Influence of protein kinase C on transcription of the tight junction elements ZO-1 and occludin. J Cell Physiol. 2005;204:83-86. [PubMed] [DOI] |
| 33. | Zhong Y, Saitoh T, Minase T, Sawada N, Enomoto K, Mori M. Monoclonal antibody 7H6 reacts with a novel tight junction-associated protein distinct from ZO-1, cingulin and ZO-2. J Cell Biol. 1993;120:477-483. [PubMed] [DOI] |
| 34. | Yang R, Harada T, Li J, Uchiyama T, Han Y, Englert JA, Fink MP. Bile modulates intestinal epithelial barrier function via an extracellular signal related kinase 1/2 dependent mechanism. Intensive Care Med. 2005;31:709-717. [PubMed] [DOI] |