修回日期: 2009-03-08
接受日期: 2009-03-26
在线出版日期: 2009-04-28
目的: 探讨重型肝炎患者外周血干细胞因子(stem cell factor, SCF)与其肝损害程度及预后的关系.
方法: 贵州省遵义医学院第一附属医院住院的肝炎患者45例及门诊体检者15例. 肝炎患者分为急性肝炎组15例, 慢性肝炎组16例和重型肝炎组14例; 重型肝炎患者又分为好转组4例, 死亡组10例. ELISA法检测各组血清SCF水平.
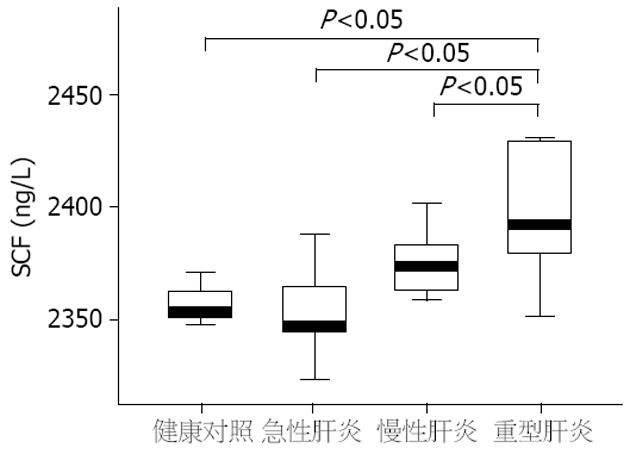
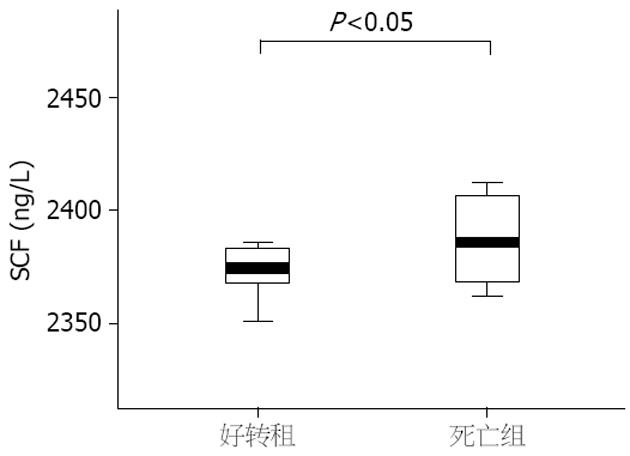
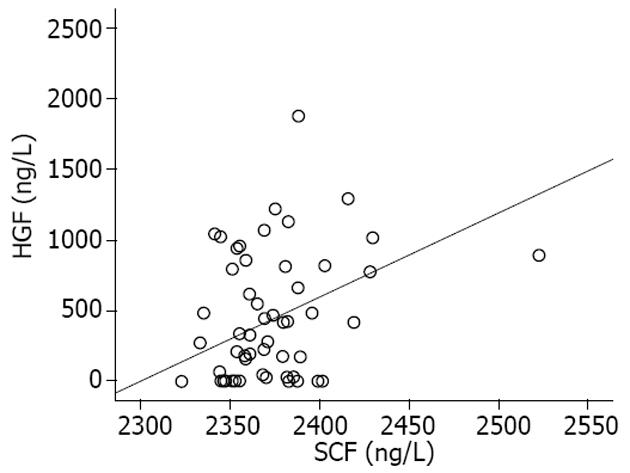
结果: 重型肝炎患者血清SCF水平明显高于急性肝炎、慢性肝炎和健康对照组, 差异具有统计学意义(2403.1±42.8 ng/L vs 2354.9±19.0 ng/L, 2376.7±16.4 ng/L, 2358.4±16.0 ng/L, 均P<0.05). 重型肝炎患者死亡组血清SCF水平明显高于好转组, 差异有统计学意义(2418.1±50.7 ng/L vs 2376.3±11.7 ng/L, P<0.05). 血清SCF与肝细胞生长因子有明显正相关(r = 0.38, P<0.01). SCF与血清白蛋白、凝血酶原时间活动度呈明显负相关(P<0.01).
结论: 重型肝炎患者外周血清SCF水平随着肝损害程度加重而明显升高, 提示严重肝损害时, 肝再生可能需要干细胞的参与.
引文著录: 刘军英, 林世德, 苏毅, 龙骏, 黄晓刚. 重型肝炎患者外周血干细胞因子水平及临床意义. 世界华人消化杂志 2009; 17(12): 1264-1268
Revised: March 8, 2009
Accepted: March 26, 2009
Published online: April 28, 2009
AIM: To investigate the serum levels of stem cell factor (SCF) and their clinical significances in patients with severe hepatitis.
METHODS: A total of 45 hepatitis patients (including 15 cases of acute hepatitis, 16 cases of chronic hepatitis and 14 cases of severe hepatitis) and 15 healthy subjects (as controls) were collected from our hospital . The severe hepatitis patients were divided into survival subgroup (n = 4) and death subgroup (n = 10). Serum levels of SCF were measured by enzyme-linked immunosorbent assay (ELISA).
RESULTS: The levels of serum SCF were significantly higher in severe hepatitis than those in acute hepatitis, chronic hepatitis and the controls (2403.1 ± 42.8 ng/L vs 2354.9 ± 19.0 ng/L, 2376.7 ± 16.4 ng/L, 2358.4 ± 16.0 ng/L; all P < 0.05), and also higher in the death subgroup than those in the survival subgroup (2418.1 ± 50.7 ng/L vs 2376.3 ± 11.7 ng/L, P < 0.05). The levels of serum SCF showed significantly positive correlation (r = 0.38, P < 0.01) with those of hepatocyte growth factor (HGF) and significantly negative correlation with those of albumin and prothrombin time activity (PTA).
CONCLUSION: Serum levels of SCF may reflect the degree of hepatic injury and the prognosis of severe hepatitis patients, indicating that stem cells may be required for liver regeneration in severe hepatitis patients.
- Citation: Liu JY, Lin SD, Su Y, Long J, Huang XG. Serum levels of stem cell factor and their clinical significances in patients with severe hepatitis. Shijie Huaren Xiaohua Zazhi 2009; 17(12): 1264-1268
- URL: https://www.wjgnet.com/1009-3079/full/v17/i12/1264.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v17.i12.1264
重型肝炎在我国较常见, 临床上重型肝炎患者虽经内科积极综合治疗, 死亡率仍高达60%-80%[1]. 肝移植是目前治疗重型肝炎最有效的方法, 但因供体肝脏来源短缺、免疫排斥反应及高额的费用等使肝移植在我国现阶段不可能成为重型肝炎患者的常规治疗方法. 因此, 提高重型肝炎患者的内科治愈率非常必要. 越来越多的研究表明重型肝炎患者死亡率居高不下的主要原因是其存在持续肝细胞凋亡和肝再生障碍, 阻止肝细胞凋亡及提高肝再生能力是提高重型肝炎患者生存率的关键[2-3]. 重型肝炎肝再生障碍的原因尚不清楚, 目前多数学者认为在重型肝炎患者肝细胞增殖有障碍的情况下, 肝再生需要干细胞的参与. 经过不断深入的研究发现, 肝细胞、卵圆细胞和肝脏前体细胞、骨髓干细胞在肝再生中有重要作用. 近来发现, 干细胞因子(stem cell factor, SCF)可以促进肝细胞增殖并可诱导和分化骨髓来源的干细胞分化为卵圆细胞和肝脏前体细胞[4]. 提示SCF在重型肝炎肝再生过程中有非常重要的作用. 但对重型肝炎患者血清SCF水平及与肝损害程度及预后的关系尚不清楚. 本研究观察重型肝炎患者血清SCF水平, 并与急性肝炎和慢性肝炎患者比较, 旨在探讨肝炎患者外周血清SCF水平与肝脏损害程度和预后的关系及其与血清肝细胞生长因子(hepatocyte growth factor, HGF)水平相关性.
2008-01/2008-09贵州省遵义医学院第一附属医院住院的肝炎患者45例及门诊体检者15例. 肝炎的诊断符合2000年西安全国传染病与寄生虫病学术会议修订的诊断标准[5]. 各组分别为健康对照组15例, 急性肝炎组15例, 慢性肝炎组16例, 重型肝炎组14例. 其中, 健康对照组为门诊体检肝功能正常者, 急性肝炎组包括甲型肝炎患者3例、乙型肝炎患者6例、甲型合并乙型肝炎患者2例、戊型合并乙型肝炎患者2例、非常见嗜肝病毒感染患者1例、病因不明的肝炎患者1例, 慢性肝炎组包括乙型肝炎患者14例, 乙型肝炎合并酒精性肝炎患者2例, 重型肝炎组包括乙型肝炎患者11例、乙型肝炎合并药物性肝炎患者1例、乙型肝炎合并酒精性肝炎患者1例, 病因不明的肝炎患者1例. 急性肝炎和慢性肝炎组患者全部病情好转出院, 14例重型肝炎患者4例病情好转出院, 10例病情恶化死亡.
1.2.1 标本采集: 健康对照组标本为门诊体检肝功能正常者的早晨空腹外周静脉血, 肝炎患者标本均为住院后第2天早晨空腹外周静脉血, 采血后5 h内离心, 转速为1000 r/min, 10 min, 然后取血清分装入Appendoff管, -35℃冰箱保存.
1.2.2 一般治疗: 所有患者住院前未接受正规治疗, 入院后予保肝, 降酶等对症治疗, 急性肝炎患者未抗病毒治疗, 慢性肝炎和重型肝炎据病情予干扰素或核苷类药物抗病毒治疗, 均未用糖皮质激素等特殊治疗.
1.2.3 血清SCF、HGF检测: SCF试剂盒购自美国ADL公司, HGF试剂盒购自美国R&D公司, ELISA法检测各组血清标本SCF、HGF水平.
统计学处理 应用SPSS13.0软件统计分析. 血清SCF和HGF水平各组用mean±SD表示, 用一维方差分析进行组间比较, t检验进行组内比较, 并进行直线相关分析.
各组间男女比例和年龄差别统计学无意义. 血常规的白细胞计数(WBC)和血小板计数(PLT)在各组间也无统计学意义. 肝功、凝血功能和甲胎蛋白(AFP)统计学结果如下表1.
重型肝炎组患者血清SCF水平明显高于急性肝炎、慢性肝炎和健康对照组(2403.1±42.8 ng/L vs 2354.9±19.0 ng/L、2376.7±16.4 ng/L、2358.4±16.0 ng/L, P<0.05, 图1). 急性肝炎、慢性肝炎和健康对照组及急性肝炎和慢性肝炎之间无明显差异. 重型肝炎组患者血清HGF水平明显高于急性肝炎、慢性肝炎和健康对照组(952.2±491.4 ng/L vs 321.1±423.9 ng/L、435.8±395.2 ng/L、76.1±126.4 ng/L, P<0.05), 急性肝炎、慢性肝炎和健康对照组及急性肝炎和慢性肝炎组之间无明显差异. 在重型肝炎患者中, 死亡组患者血清SCF水平明显高于好转组, 两者之间有统计学意义(2376.3±11.7 ng/L vs 2418.1±50.7 ng/L P<0.05, 图2), 而死亡组患者血清HGF与好转组之间无明显差异(551.3±189.1 ng/L vs 607.1±213.3 ng/L, P>0.05).
2.3 血清SCF、HGF水平的相关性 各组间血清SCF与HGF有明显的正相关(r = 0.38, P<0.01, 图3).
2.4 血清SCF水平与血清生化指标之间的相关性 血清SCF与血清白蛋白、凝血酶原时间活动度和纤维蛋白原有明显负相关(P<0.01), 与凝血酶原时间比值和活化部分凝血酶时间呈明显正相关(P<0.01). 各组血清SCF与血生化指标的相关性如下表2.
| 生化指标 | SCF | HGF | ||
| 相关系数 | P值 | 相关系数 | P值 | |
| ALT(U/L) | 0.000 | 无意义 | 0.413 | <0.01 |
| TB(μmol/L) | 0.274 | 无意义 | 0.430 | <0.01 |
| ALB(g/L) | -0.422 | <0.01 | -0.429 | <0.01 |
| PTA(%) | -0.466 | <0.01 | -0.548 | <0.01 |
| PTR | 0.449 | <0.01 | 0.627 | <0.01 |
| APTT(s) | 0.481 | <0.01 | 0.549 | <0.01 |
| FIB(g/L) | -0.517 | <0.01 | -0.361 | <0.05 |
| TT(s) | 0.087 | 无意义 | 0.236 | 无意义 |
| AFP(U/L) | 0.035 | 无意义 | 0.004 | 无意义 |
| WBC(×109/L) | 0.191 | 无意义 | 0.635 | <0.01 |
| PLT(×109/L) | -0.076 | 无意义 | 0.081 | 无意义 |
正常情况下, 启动成熟肝细胞快速分裂增殖是肝脏生长和修复的一种反应. 近几年的研究表明, 当肝脏损伤严重致肝细胞大量坏死或肝细胞再生受到抑制时, 肝门束周围出现大量卵圆细胞, 参与肝脏的损伤和修复, 成为肝再生的主要来源, 这种细胞被认为是肝脏干细胞[6-7]. Petersen et al[8]首先用动物模型进行了骨髓移植实验, 提出骨髓干细胞可转化为肝卵圆细胞, 此后, Alison et al[9]和Theise et al[10]分别报道, 人类异性骨髓和肝移植, 在受者肝细胞内发现了供体的染色体. 体内外[11-13]实验均发现骨髓干细胞可诱导成肝脏干细胞和肝细胞.
大量的生长因子和细胞因子参与了肝再生, SCF是一种作用于最早期造血干细胞的造血因子. 研究表明SCF是一个强有力的休眠干细胞的诱导物, 在维持造血细胞存活, 刺激骨髓的造血干细胞增殖分化生长和迁徙到外周血液循环中起重要作用[14]. 大量数据显示了SCF在多种细胞的成熟和增殖中有更广泛的作用, 在肝脏受损时SCF明显表达, 可能作为肝细胞的一种增生诱导物, 诱导骨髓来源的干细胞分化为肝卵圆细胞, 并在卵圆细胞的发育中有重要作用[4,15-17]. 肝脏70%切除后, 注入anti-SCF抗体的小鼠以及SCF基因缺乏的小鼠, 其肝脏再生的速度要比正常分泌SCF的肝脏要慢得多, 将SCF注射到SCF基因缺乏小鼠后, 其肝脏能以接近正常的速度再生[4]. SCF的受体是c-kit, 后者是一种原癌基因, 在许多肿瘤组织其中包括肝母细胞瘤都有SCF和c-kit表达, 在肿瘤的发生发展和预后中有重要的作用[18-20]. 在扑热息痛诱导的急性肝衰竭模型中, SCF和c-kit mRNA表达升高, 外源性的SCF注入增加了肝细胞增殖并抑制肝细胞凋亡且减少了致死率[21-22]. 这些均提示SCF与肝再生关系密切.
HGF是被公认的成熟肝细胞最强有力的有丝分裂原, 并能促进未活化的肝细胞和卵圆细胞分化[23-25]. 本研究显示重型肝炎患者血清SCF水平明显高于急性肝炎, 慢性肝炎和健康对照组, 血清HGF水平在重型肝炎升高明显, 且血清HGF、SCF有明显正相关, 与PT呈显著负相关, 死亡组患者血清SCF明显高于好转组, 提示血清SCF及HGF水平与肝损害程度有关, 随着肝损害程度的加重, 血清SCF及HGF水平升高. 重型肝炎血清SCF水平升高的原因目前尚不清楚, 可能在重型肝炎患者肝细胞广泛损伤、肝细胞的增殖有障碍的情况下, 肝损伤的修复需要干细胞的参与, 而高水平的血清SCF更有利于干细胞向肝细胞增殖分化.
重型肝炎患者肝脏大面积损伤及肝再生障碍与很多因素有关[26], 因而, 重型肝炎患者的预后与肝损害程度、病因、病程、治疗方法等多种因素有关, 临床上很难用一两个生化指标准确的判断重型肝炎患者的预后. 本研究结果提示重型肝炎死亡组患者血清SCF水平明显高于生存组, 而HGF水平无明显差异, 提示重型肝炎患者血清SCF水平比HGF水平能更准确反映其肝损害程度, 并与预后相关. 关于SCF分泌和作用机制及其在临床的应用价值仍待进一步探讨.
重型肝炎在我国较常见, 临床上内科综合治疗非常有限, 肝移植因其诸多弊端难以推广. 如何提高肝再生能力成为提高重型肝炎患者生存率的关键. 干细胞因子是1990年发现的一种重要造血干细胞生成因子. 近年来发现他与肝细胞再生关系密切而备受关注.
刘杞, 教授, 重庆医科大学病毒性肝炎研究所; 宁琴, 教授, 华中科技大学同济医学院附属同济医院感染科; 党双锁, 教授, 西安交通大学第二医院感染科
近来发现, 干细胞因子可以促进肝细胞增殖并可诱导和分化骨髓来源的干细胞分化为卵圆细胞和肝脏前体细胞. 提示SCF在肝再生过程中有非常重要的作用.
Avital et al研究发现在体内外骨髓干细胞均可分化为具有肝细胞表型和功能的细胞. Fujio et al用三种动物模型检测其血清SCF水平, 发现AAF注入并部分肝切除模型血清SCF水平升高最明显. Ramsfjell et al认为SCF可诱导骨髓干细胞进入外周循环.
重型肝炎患者血清SCF水平比HGF水平能更准确反映其肝损害程度, 并与预后相关.
本文新颖性较强, 层次分明, 实用性强, 对临床医师有一定的指导意义.
编辑: 李军亮 电编:何基才
| 1. | Bathgate AJ, Garden OJ, Forsythe JR, Madhaven KK, Finlayson ND, Simpson KJ, Hayes PC, MacGilchrist AJ. The outcome of the first 165 orthotopic liver transplants in Scotland. Scott Med J. 1999;44:9-10. [PubMed] |
| 3. | Lin SD, Kawakami T, Ushio A, Sato A, Sato S, Iwai M, Endo R, Takikawa Y, Suzuki K. Ratio of circulating follistatin and activin A reflects the severity of acute liver injury and prognosis in patients with acute liver failure. J Gastroenterol Hepatol. 2006;21:374-380. [PubMed] [DOI] |
| 4. | Ren X, Hogaboam C, Carpenter A, Colletti L. Stem cell factor restores hepatocyte proliferation in IL-6 knockout mice following 70% hepatectomy. J Clin Invest. 2003;112:1407-1418. [PubMed] |
| 6. | Pack R, Heck R, Dienes HP, Oesch F, Steinberg P. Isolation, biochemical characterization, long-term culture, and phenotype modulation of oval cells from carcinogen-fed rats. Exp Cell Res. 1993;204:198-209. [PubMed] [DOI] |
| 7. | Yasui O, Miura N, Terada K, Kawarada Y, Koyama K, Sugiyama T. Isolation of oval cells from Long-Evans Cinnamon rats and their transformation into hepatocytes in vivo in the rat liver. Hepatology. 1997;25:329-334. [PubMed] |
| 8. | Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase N, Boggs SS, Greenberger JS, Goff JP. Bone marrow as a potential source of hepatic oval cells. Science. 1999;284:1168-1170. [PubMed] [DOI] |
| 9. | Alison MR, Poulsom R, Jeffery R, Dhillon AP, Quaglia A, Jacob J, Novelli M, Prentice G, Williamson J, Wright NA. Hepatocytes from non-hepatic adult stem cells. Nature. 2000;406:257. [PubMed] [DOI] |
| 10. | Theise ND, Nimmakayalu M, Gardner R, Illei PB, Morgan G, Teperman L, Henegariu O, Krause DS. Liver from bone marrow in humans. Hepatology. 2000;32:11-16. [PubMed] [DOI] |
| 11. | Okumoto K, Saito T, Hattori E, Ito JI, Adachi T, Takeda T, Sugahara K, Watanabe H, Saito K, Togashi H. Differentiation of bone marrow cells into cells that express liver-specific genes in vitro: implication of the Notch signals in differentiation. Biochem Biophys Res Commun. 2003;304:691-695. [PubMed] [DOI] |
| 12. | Okumoto K, Saito T, Hattori E, Ito JI, Suzuki A, Misawa K, Sanjyo M, Takeda T, Sugahara K, Saito K. Expression of Notch signalling markers in bone marrow cells that differentiate into a liver cell lineage in a rat transplant model. Hepatol Res. 2005;31:7-12. [PubMed] [DOI] |
| 13. | Okumoto K, Saito T, Hattori E, Ito JI, Suzuki A, Misawa K, Ishii R, Karasawa T, Haga H, Sanjo M. Differentiation of rat bone marrow cells cultured on artificial basement membrane containing extracellular matrix into a liver cell lineage. J Hepatol. 2005;43:110-116. [PubMed] [DOI] |
| 14. | McNiece IK, Briddell RA, Hartley CA, Andrews RG. The role of stem cell factor in mobilization of peripheral blood progenitor cells: synergy with G-CSF. Stem Cells. 1993;11 Suppl 3:83-88. [PubMed] |
| 15. | Matsusaka S, Tsujimura T, Toyosaka A, Nakasho K, Sugihara A, Okamoto E, Uematsu K, Terada N. Role of c-kit receptor tyrosine kinase in development of oval cells in the rat 2-acetylaminofluorene/partial hepatectomy model. Hepatology. 1999;29:670-676. [PubMed] [DOI] |
| 16. | Uchida N, Dykstra B, Lyons KJ, Leung FY, Eaves CJ. Different in vivo repopulating activities of purified hematopoietic stem cells before and after being stimulated to divide in vitro with the same kinetics. Exp Hematol. 2003;31:1338-1347. [PubMed] [DOI] |
| 17. | Baccarani U, De Stasio G, Adani GL, Donini A, Sainz-Barriga M, Lorenzin D, Beltrami A, Bresadola V, Risaliti A, Bresadola F. Implication of stem cell factor in human liver regeneration after transplantation and resection. Growth Factors. 2006;24:107-110. [PubMed] [DOI] |
| 18. | Matsui J, Yamamoto Y, Funahashi Y, Tsuruoka A, Watanabe T, Wakabayashi T, Uenaka T, Asada M. E7080, a novel inhibitor that targets multiple kinases, has potent antitumor activities against stem cell factor producing human small cell lung cancer H146, based on angiogenesis inhibition. Int J Cancer. 2008;122:664-671. [PubMed] [DOI] |
| 19. | Kartha RV, Sundram UN. Silent mutations in KIT and PDGFRA and coexpression of receptors with SCF and PDGFA in Merkel cell carcinoma: implications for tyrosine kinase-based tumorigenesis. Mod Pathol. 2008;21:96-104. [PubMed] |
| 20. | Stoop H, Honecker F, van de Geijn GJ, Gillis AJ, Cools MC, de Boer M, Bokemeyer C, Wolffenbuttel KP, Drop SL, de Krijger RR. Stem cell factor as a novel diagnostic marker for early malignant germ cells. J Pathol. 2008;216:43-54. [PubMed] [DOI] |
| 21. | Simpson K, Hogaboam CM, Kunkel SL, Harrison DJ, Bone-Larson C, Lukacs NW. Stem cell factor attenuates liver damage in a murine model of acetaminophen-induced hepatic injury. Lab Invest. 2003;83:199-206. [PubMed] |
| 22. | Hu B, Colletti LM. Stem cell factor and c-kit are involved in hepatic recovery after acetaminophen-induced liver injury in mice. Am J Physiol Gastrointest Liver Physiol. 2008;295:G45-G53. [PubMed] [DOI] |
| 23. | Hamamoto R, Kamihira M, Iijima S. Growth and differentiation of cultured fetal hepatocytes isolated various developmental stages. Biosci Biotechnol Biochem. 1999;63:395-401. [PubMed] [DOI] |
| 24. | Okano J, Shiota G, Matsumoto K, Yasui S, Kurimasa A, Hisatome I, Steinberg P, Murawaki Y. Hepatocyte growth factor exerts a proliferative effect on oval cells through the PI3K/AKT signaling pathway. Biochem Biophys Res Commun. 2003;309:298-304. [PubMed] [DOI] |
| 25. | Hasuike S, Ido A, Uto H, Moriuchi A, Tahara Y, Numata M, Nagata K, Hori T, Hayashi K, Tsubouchi H. Hepatocyte growth factor accelerates the proliferation of hepatic oval cells and possibly promotes the differentiation in a 2-acetylaminofluorene/partial hepatectomy model in rats. J Gastroenterol Hepatol. 2005;20:1753-1761. [PubMed] [DOI] |