修回日期: 2008-01-04
接受日期: 2008-01-15
在线出版日期: 2008-02-08
目的: 探讨血小板活化因子(PAF)是否会引起肠黏膜屏障的破坏以及这种破坏机制是否与紧密连接相关, 并观察肠三叶因子(ITF)的保护作用.
方法: 培养人结肠腺癌细胞株Caco-2, 分别设正常对照组: 不加刺激物及干预因素; 实验组: 加入PAF, 终浓度分别为50 ng/L、100 ng/L和200 ng/L; ITF预防组: 先加入ITF0.3 g/L, 30 min后加入PAF 100 ng/L; ITF治疗组: 先加入PAF 100 ng/L, 30 min后加入ITF0.3 g/L, 24 h后进行实验. MTT比色法检测细胞活力; 测定跨上皮电阻TER和荧光黄的透过量反映肠上皮细胞单层通透性; RT-PCR法检测紧密连接蛋白ZO-1和Occludin mRNA表达的变化; 免疫荧光染色观察紧密连接蛋白ZO-1和Occludin的形态学.
结果: PAF未影响到细胞的增殖和细胞活力. 各浓度PAF作用24 h后, 细胞单层通透性增加, 跨上皮电阻(TER)下降, 荧光黄透过增加. ITF治疗组及预防组TER下降值较模型组明显降低(122.2±14.7, 100.3±10.9 vs 210.3±26.4, P<0.05), 荧光黄透过量减少(10226.1±556.2, 9711.2±364.9 vs 11601.2±693.5, P<0.05), 预防组作用更明显. PAF作用后, ZO-1和Occludin mRNA 表达均有下降, 以100 ng/L组改变最为明显, ITF预防组较之表达增加(1.28±0.06 vs 1.07±0.05, 1.13±0.07 vs 0.81±0.06, P<0.05), ITF治疗组改变不明显. 免疫荧光染色发现ZO-1和Occludin主要分布在细胞内近胞膜处. PAF作用后, ZO-1及Occludin形态学改变, 预防性给予ITF后, 可明显减轻这种改变.
结论: PAF可引起肠黏膜屏障破坏, 其机制可能和紧密连接蛋白的破坏相关; ITF可以通过改变紧密连接蛋白的表达而部分恢复肠黏膜正常通透性, 起到保护作用.
引文著录: 许玲芬, 董亚珞, 孙梅, 马力, 毛志芹. PAF对肠上皮细胞紧密连接的影响及ITF的保护作用. 世界华人消化杂志 2008; 16(4): 372-378
Revised: January 4, 2008
Accepted: January 15, 2008
Published online: February 8, 2008
AIM: To explore whether platelet-activating factor (PAF) can disrupt the intestinal epithelial barrier and is associated with tight junction, and to observe the protective effect of intestinal trefoil factor (ITF).
METHODS: Caco-2 cells were cultured with RPMI 1640 containing 15% fetal bovine serum for 7 days. Cells were then treated with different concentrations of PAF (0, 50, 100 and 200 ng/L) for 24 hours until they became fused. The protective effect of ITF was observed. rITF (0.3 g/L) was treated 30 minutes before (prevention group) and after PAF (treat proup) was given and cells were incubated for 24 hours. MTT was used to detect cell vigor. Epithelial monolayer permeability was measured by trans-epithelial electrical resistance (TER) and mucosal to serosal flux of paracellular marker luminal yellow. RT-PCR was used to detect mRNA expression of ZO-1 and Occludin. Indirect immunofluorescence was used to localize ZO-1 and Occludin.
RESULTS: PAF had no effect on the Caco-2's vigor and proliferation. Different concentrations of PAF increased the intestinal epithelial paracellular permeability. TER decreased and luminal yellow flux increased. Treatment and prevention group of rITF partially recovered the increased permeability (122.2 ± 14.7, 100.3 ± 10.9 vs 210.3 ± 26.4, P < 0.05; 10226.1 ± 556.2, 9711.2 ± 364.9 vs 11601.2 ± 693.5, P < 0.05). RT-PCR demonstrated that the mRNA expression of ZO-1 and Occludin in PAF groups treated with different concentrations (especilly 100 ng/L) for 24 hours was significantly different from that in the prevention group (1.07 ± 0.05 vs 1.28 ± 0.06, 0.81 ± 0.06 vs 1.13 ± 0.07, P < 0.05). However, treatment of rITF did not change the expression of mRNA. In the control Caco-2 monolayers, ZO-1 and Occludin proteins were localized at the apical cellular junctions and appeared as continuous belt-like structures encircling the cells at the cellular borders. PAF caused a progressive disturbance in the continuity of ZO-1 and Occludin localization at the cellular borders characterized by zig-zagging appearance at points of multiple cellular contact. rITF could partially recover the disrupted distribution of ZO-1 and Occludin proteins.
CONCLUSION: PAF disrupts the intestinal epithelial barrier. Its mechanism may be correlated to the decreased expression of ZO-1 and Occludin proteins, which can be partially recovered after treatment of rITF.
- Citation: Xu LF, Dong YL, Sun M, Ma L, Mao ZQ. Effect of platelet-activating factor on tight junction in intestinal epithelial cells and protective effect of intestinal trefoil factor. Shijie Huaren Xiaohua Zazhi 2008; 16(4): 372-378
- URL: https://www.wjgnet.com/1009-3079/full/v16/i4/372.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v16.i4.372
肠黏膜屏障的破坏在儿科急慢性疾病的发生发展中具有显著的意义[1-4]: 急性期, 细菌移位可引起败血症及多器官功能衰竭, 而婴儿时期的肠黏膜屏障破坏将会成为随后产生的部分变应性疾病的始发因素, 如: 湿疹, 食物过敏, 腹部肠病, 1型糖尿病, 哮喘, 炎症性肠病及孤独症等. 而肠黏膜通透性的升高是早期和主要的病理生理基础. 紧密连接作为细胞间通路最主要的屏障直接影响肠黏膜屏障的通透性[5-6].
血小板活化因子(PAF)是一种内源性脂性介质, 目前被认为是一种独特的细胞因子, 具有广泛的生物学特性, 研究认为[7]PAF在诸多参与胃肠黏膜损害的炎症介质中可能起到"中心放大"的介导作用. 动物实验也注意到PAF可引起肠黏膜通透性的增高[8]. 已有实验证实[9], 用PAF和LPS引起的坏死性小肠结肠炎的改变相似, 用PAF受体拮抗剂可明显减轻LPS所致的肠坏死[10]. 但其机制是否与紧密连接的破坏相关尚无报道.
肠三叶因子(ITF)属三叶肽家族, 因其对胃肠道黏膜屏障有重要保护和修复作用而受到越来越多的关注. 大量的动物实验证明[11-13]ITF在维持肠上皮细胞的完整性, 恢复肠黏膜的正常通透性方面起到重要作用. 本研究试图在细胞水平观察ITF对紧密连接的保护作用从而为临床应用提供依据.
人结肠腺癌细胞株(Caco-2)购于中国科学院上海细胞所; RPMI 1640培养基、胰酶和胎牛血清(fatal bovine serum, FBS)购自Gibco公司; PAF、荧光黄购自Sigma公司; ZO-1、Occludin单抗、异硫氰酸荧光素(fluorescinisothiocyate, FITC)标记羊抗兔IgG购自Zymed公司; rITF(基因重组肠三叶因子)由北京大学蛋白质工程及植物基因工程国家重点实验室提供; MTT和DMSO为AMRECO产品; transwell购自美国Corning公司; 电阻仪购自美国Millipore公司; RT-PCR试剂盒购自TaKaRa公司; 引物也由该公司合成.
1.2.1 细胞培养条件及方法: Caco-2细胞常规培养, 培养液为RPMI 1640培养基, 含150 mL/L进口胎牛血清, 10 mL/L青链霉素双抗液, 通入50 mL/L CO2(相对湿度90%), 置37 ℃培养箱, 传代后在细胞生长达到各项实验要求时进行实验, 每项实验均选取3组非同代细胞进行.
1.2.2 实验分组: 分别设正常对照组、实验组(含模型对照组)、ITF预防组、ITF治疗组. 正常对照组: 不加刺激物及干预因素. 实验组: 加入PAF, 终浓度分别为50 ng/L、100 ng/L和200 ng/L, 并把终浓度100 ng/L的组作为模型对照组, 刺激24 h. ITF预防组: 先加入ITF 0.3 g/L, 30 min后加入PAF 100 ng/L, 24 h后进行实验. ITF治疗组: 先加入PAF 100 ng/L, 30 min后加入ITF 0.3 g/L, 24 h后进行实验.
1.2.3 血细胞活力检测(MTT比色法): 取对数生长期Caco-2细胞, 消化后加入一定量的培养液, 制成细胞悬液, 按密度为5000细胞/(100 μL·孔)接种在96孔板, 通入50 mL/L CO2(相对湿度90%), 置37 ℃培养箱中进行培养. 24 h细胞贴壁融合后, 换无血清培养液, 加入不同浓度PAF(0, 10, 20, 40, 80, 160, 320, 640, 1280, 2560, 5120 ng/L), 每个浓度设4个复孔继续培养24 h, 加入5 g/L MTT 20 μL/孔, 继续培养4 h, 吸去培养液, 加入150 μL/孔DMSO, 振荡10 min, 用酶标仪在492 nm处测定吸光度.
1.2.4 肠上皮细胞单层通透性: 跨上皮电阻(TER)的测定: 肠上皮细胞Caco-2以105/cm2接种于transwell的顶层小室微孔膜上(直径6.5 mm, 孔径大小0.4 mm)培养, 待细胞长至融合, 形成致密的单层屏障后开始实验(10-14 d). 先将培养液换成无血清培养液平衡0.5 h, 然后用电阻仪测定TER, 每个transwell测定3个不同位置, 记录电阻值(Ω/cm2). 按照实验分组于transwell底层小室加入PAF及ITF, 加药后24 h分别测电阻. 荧光黄测定: 加药后24 h, 细胞用HBSS(pH7.4)小心冲洗3次, 37 ℃孵育30 min, 吸净孔内的HBSS以防止干扰; 在transwell顶端加入80 mg/L荧光黄, 37 ℃孵育1 h后收集基底侧液体, 4 ℃避光保存, 用荧光分光光度计测定荧光黄浓度(激发波长427 nm, 发射波长536 nm)
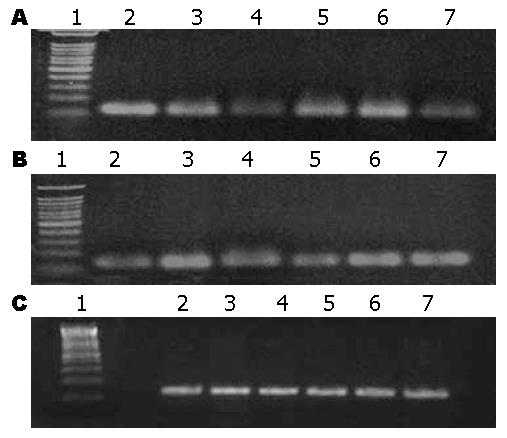
1.2.5 紧密连接蛋白ZO-1和Occludin mRNA表达的变化: 于50 mL细胞培养瓶中培养细胞至接近融合时换无血清培养液, 按照实验分组加入PAF及ITF, 24 h后收集细胞. TRIzol提取总RNA, 行RT-PCR反应, 反应条件: 94 ℃ 2 min; 94 ℃ 30 s; 58 ℃ 30 s; 72 ℃ 30 s, 32个循环. 扩增产物经琼脂糖凝胶电泳, 紫外灯下观察RT-PCR产物并拍照, 用Kodak ID型凝胶成像系统输入计算机, 以该软件进行扩增产物的半定量分析. ZO-1引物: 上游: 5'-AGCCTGCAAAGCCAGCTCA-3', 下游: 5'-AGTGGCCTGGATGGGTTCATAG-3'; 扩增片段131 bp. Occludin引物: 上游: 5'-AAGAGTTGACAGTCCCATGGCATAC-3', 下游: 5'-ATCCACAGGCGAAGTTAATGGAAG-3'; 扩增片段133 bp. GAPDH引物: 上游: 5'-GCACCGTCAAGGCTGAGAAC-3', 下游: 5'-ATGGTGGTGAAGACGCCAGT-3'; 扩增片段142 bp.
1.2.6 紧密连接蛋白ZO-1和Occludin免疫荧光染色: 置盖玻片于六孔板中接种细胞爬片生长至融合, 换无血清培养液, 按照实验分组加入PAF及ITF, 刺激24 h. 弃培养液, PBS洗涤, 40 g/L多聚甲醛固定, 0.5% Triton X-100打孔, 10% BSA封闭, 加一抗4 ℃过夜(1:50), 加二抗(1:100)避光染色2 h后甘油封片, 用荧光显微镜观察并拍照.
统计学处理 所有实验均重复3次以上, 结果用mean±SD表示, 统计由SPSS12.0软件完成. 采用One-Way ANOVA法比较总体和组间差异, P<0.05为有显著差异.
MTT还原物甲攒是细胞能量代谢的反映, 其生成量随细胞数的增多而增加. 由表1可以看出, 即使大剂量的PAF也未影响到细胞的增殖和细胞活力, 各加药浓度与对照组相比无显著性差异.
| 分组 | PAF给药浓度(ng/L) | A值 | P值 |
| Control | 0 | 0.7803±0.0078 | |
| 1 | 5120 | 0.7893±0.2577 | 1.000 |
| 2 | 2560 | 0.8073±0.3150 | 0.674 |
| 3 | 1280 | 0.7917±0.2329 | 0.997 |
| 4 | 640 | 0.7730±0.1418 | 1.000 |
| 5 | 320 | 0.7713±0.0839 | 1.000 |
| 6 | 160 | 0.7653±0.3539 | 0.979 |
| 7 | 80 | 0.7623±0.1602 | 0.939 |
| 8 | 40 | 0.7707±0.1732 | 0.999 |
| 9 | 20 | 0.7573±0.1217 | 0.812 |
| 10 | 10 | 0.8063±0.0027 | 0.710 |
各浓度PAF作用24 h后, TER出现下降, 荧光黄透过增加. 给予ITF可明显提高TER, 减少荧光黄透过. 预防组作用更明显(表2).
PAF作用后, ZO-1和Occludin mRNA表达均有下降, 以100 ng/L组改变最为明显. ITF预防组表达增加, ITF治疗组改变不明显(表3, 图1).
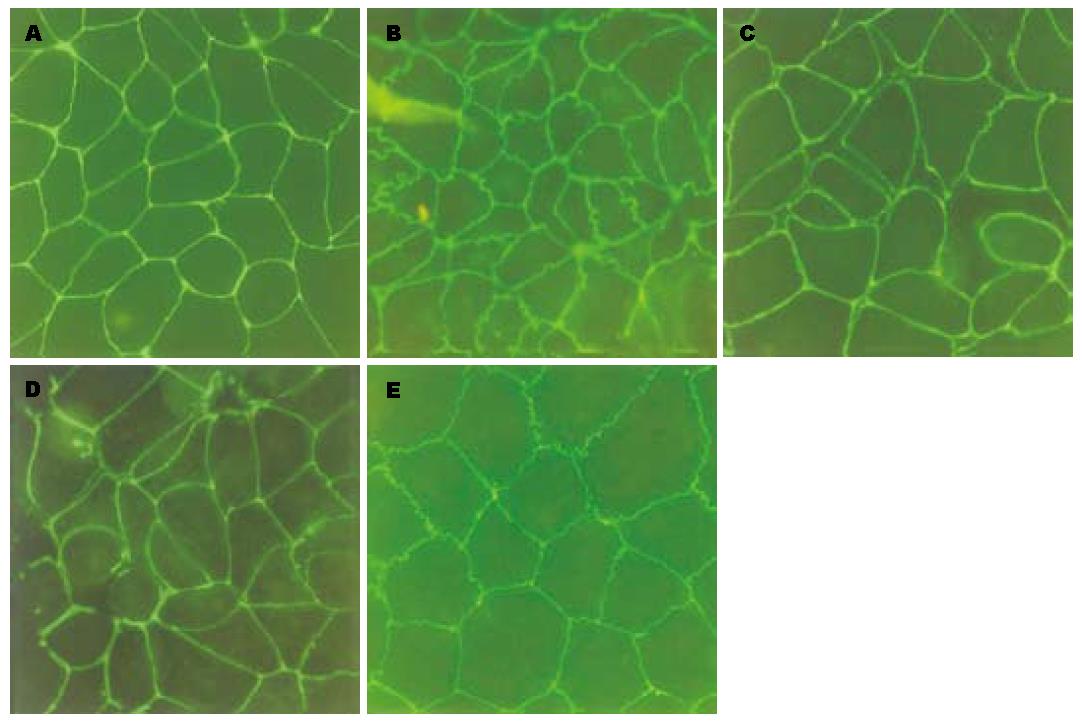
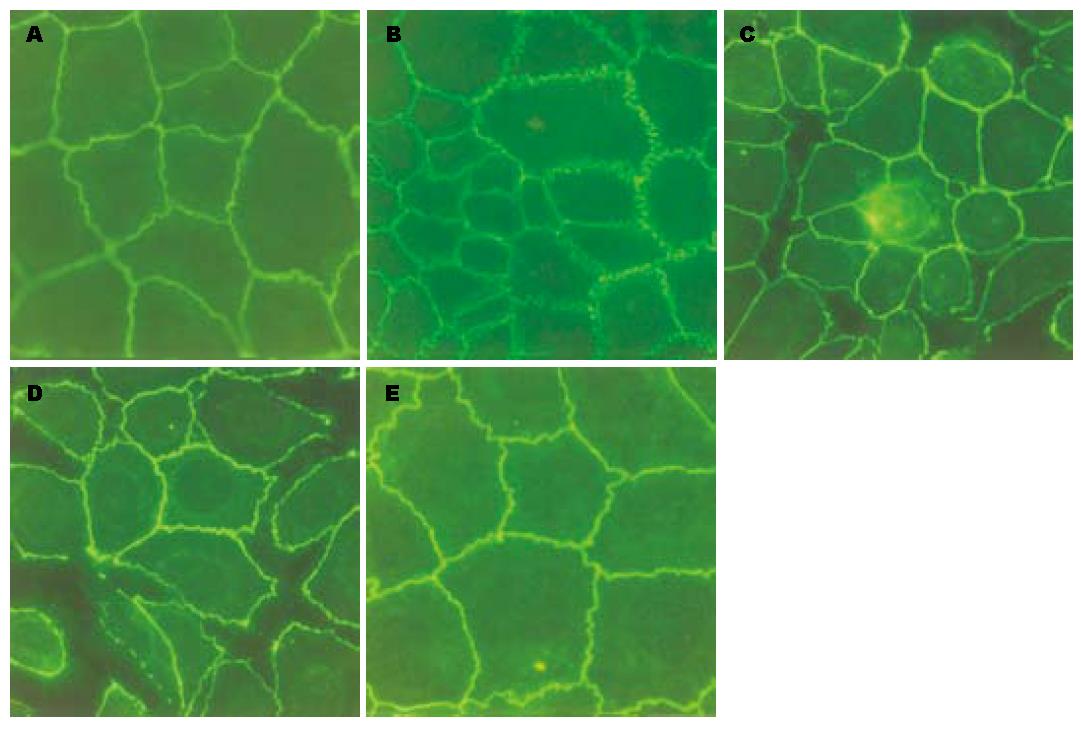
正常对照组上皮细胞ZO-1主要分布在细胞内近胞膜处, 排列紧密, 边缘光滑, 勾勒出内皮细胞典型的铺路石形状, 细胞间连接紧密, 无明显间隙. 小浓度PAF(50 ng/L)作用后, ZO-1边缘粗糙呈锯齿状, 连接处可见"蜂窝"状改变. 随PAF浓度加大(100 ng/L)改变更加明显, 细胞间出现间隙, ZO-1环断裂, 甚至崩解. 但PAF浓度继续加大, 改变并未继续加重. 预防性给予ITF后, 可明显减轻ZO-1的破坏, 边缘变得连续, 但没有完全恢复. ITF治疗组改变较预防组重. Occludin表达位置和变化规律与ZO-1基本一致(图2, 图3).
肠黏膜屏障包括肠上皮细胞屏障、免疫屏障和微生物屏障, 肠上皮细胞屏障是最重要的一道屏障, 是肠黏膜屏障具有选择性通透的基础. 目前研究发现肠上皮细胞屏障通透性增高参与多种疾病的发生, 如: 炎症性肠病[14]、脓毒血症、烧伤、终末期肝病、重症胰腺炎等. 而他在儿科急慢性疾病的发生发展中所具有的显著意义使得肠黏膜屏障功能及其完整性这一课题在儿科领域也受到越来越多的关注.
正常情况下肠上皮细胞细胞旁间隙是由连接复合体封闭的, 其中最重要的是紧密连接(tight junction, TJ). 紧密连接由多种紧密连接蛋白分子组成, 包括跨膜蛋白Occludin、Claudins、连接黏附分子(junctional adhesion molecule, JAM)和胞质附着蛋白ZOs、AF6、7H6等. Occludin是最先分离出来的TJ跨膜蛋白[15], 分子质量为65 kDa, 形成两个细胞外环和一个短的细胞内环. 免疫电镜显示Occludin定位在TJ上[16-18], 是TJ的主要功能蛋白, 对于屏障功能的维持和紧密连接的完整性具有重要作用. 有研究表明用Occludin转染L-纤维母细胞(缺乏TJ)后, 相邻细胞之间可以形成TJ样结构[19]. ZOs是一种外周膜蛋白, 有三种异构体即ZO-1, ZO-2和ZO-3, 含有PDZ等保守序列. 这些保守序列能够与细胞质内的其他蛋白如Occludins蛋白的C末端连接, 而ZO-1的C末端则可结合肌动蛋白和应力纤维, 从而将Occludin蛋白和肌动蛋白骨架系统连接在一起, 构成稳定的连接系统. ZO-1的N末端可直接与α2连锁蛋白(catenin)或细胞质E-钙黏连素结合, 介导紧密连接的开启和闭合[20-25]. 因此, Occludin和ZO-1蛋白对维持TJ的正常结构和功能具有重要意义.
既往研究发现PAF可激活巨噬细胞和中性粒细胞, 促进其黏附到血管内皮细胞, 释放氧自由基和蛋白水解酶, 破坏内皮细胞的形态和功能. 在血栓形成、休克、过敏性哮喘、内毒素血症和肠道坏死中均起重要作用[26], 但PAF是否可以直接破坏紧密连接而引起肠上皮屏障破坏、肠通透性增高尚不清楚.
Caco-2细胞来源于人的直肠癌, 在细胞培养条件下, 可融合并分化为肠上皮细胞, 形成连续的单层, 细胞亚显微结构研究表明, Caco-2细胞与人小肠上皮细胞在形态学上相似, 具有相同的细胞极性和紧密连接, 是国际上公认的研究紧密连接和药物小肠吸收的体外模型[27].
本实验应用Caco-2细胞构建肠黏膜屏障的体外模型, 观察PAF破坏肠黏膜屏障的机制, 发现给予不同浓度的PAF, 在不影响细胞活力的情况下, 已经引起了TER的降低及肠屏障通透性的升高, 而且这种改变具有一定的剂量依赖性, 在PAF浓度为100 ng/L时最强, 研究认为PAF通过PAF受体起作用, 考虑这种浓度依赖性与受体饱和性有关. 进一步的研究发现构成紧密连接最重要的两个蛋白-膜周边蛋白ZO-1和跨膜蛋白Occludin在基因和蛋白水平上均发生了改变, 推测PAF可以通过影响紧密连接蛋白的表达而影响肠上皮屏障.
ITF在肠道的自我保护和损伤后修复中占有重要地位, 其机制涉及促进细胞迁移、增殖和抗凋亡等作用. 近期研究发现, ITF高表达的细胞, 其TER明显增高, 肠通透性降低[28]. 本实验也证明ITF可以通过影响紧密连接蛋白的表达而恢复紧密连接的结构, 恢复正常的肠屏障结构, 从而发挥其保护作用.
早在20世纪80年代就有学者提出"胃肠道是MSOF的始动器官", 目前认为肠黏膜受损最早的表现是通透性增加, 而通透性增加与紧密连接的破坏密切相关. 肠三叶因子在肠道的自我保护和修复中具有重要作用, 但其机制是否与紧密连接相关尚无报道.
秦环龙, 教授, 上海交通大学附属第六人民医院外科
PAF是一种脂质类炎性介质, 很可能是内毒素作用的主要效应因子. 研究认为PAF在诸多参与胃肠黏膜损害的炎症介质中可能起到"中心放大"的介导作用. PAF引起的肠黏膜屏障通透性升高的作用机制及寻求有效的预防和治疗手段是目前研究的热点.
有报道PAF可引起肠黏膜通透性的增高, PAF和LPS引起的坏死性小肠结肠炎的改变相似, 且PAF受体拮抗剂可明显减轻LPS所致的肠坏死. 体外实验发现ITF可以调控紧密连接蛋白Claudin-1和Claudin-2的表达而影响肠上皮的跨上皮阻力.
目前关于PAF引起肠黏膜通透性增高的体外实验报道极少, 他对于肠上皮细胞间紧密连接蛋白ZO-1和Occludin表达变化的文章国内尚未见报道. 研究ITF对肠黏膜损伤的保护作用并从紧密连接的角度探讨其作用机制是本研究的创新.
本文为大家进一步深入研究PAF损伤肠黏膜屏障的机制提供了基础, 同时为ITF生物制剂的开发和应用提供了理论基础.
肠三叶因子(ITF): 属三叶肽家族, 对胃肠道黏膜屏障有重要保护和修复作用, 是一种新型的生长因子类多肽物质. 他可与黏液糖蛋白相互作用或交联, 固定和支持肠道黏液层并促进细胞增殖与移行, 在肠道的自我保护机制中占据重要地位.
本文研究具有较强的科学性和说服力.
编辑: 杜群 电编: 何基才
| 1. | Macintire DK, Bellhorn TL. Bacterial translocation: clinical implications and prevention. Vet Clin North Am Small Anim Pract. 2002;32:1165-1178. [PubMed] [DOI] |
| 2. | Lichtman SM. Bacterial [correction of baterial] translocation in humans. J Pediatr Gastroenterol Nutr. 2001;33:1-10. [PubMed] [DOI] |
| 3. | Farhadi A, Banan A, Fields J, Keshavarzian A. Intestinal barrier: an interface between health and disease. J Gastroenterol Hepatol. 2003;18:479-497. [PubMed] [DOI] |
| 4. | Berkes J, Viswanathan VK, Savkovic SD, Hecht G. Intestinal epithelial responses to enteric pathogens: effects on the tight junction barrier, ion transport, and inflammation. Gut. 2003;52:439-451. [PubMed] [DOI] |
| 5. | Cereijido M, Contreras RG, Flores-Benítez D, Flores-Maldonado C, Larre I, Ruiz A, Shoshani L. New diseases derived or associated with the tight junction. Arch Med Res. 2007;38:465-478. [PubMed] [DOI] |
| 6. | González-Mariscal L, Betanzos A, Nava P, Jaramillo BE. Tight junction proteins. Prog Biophys Mol Biol. 2003;81:1-44. [PubMed] [DOI] |
| 7. | Kalia N, Bardhan KD, Reed MW, Jacob S, Brown NJ. Mechanisms of Helicobacter pylori-induced rat gastric mucosal microcirculatory disturbances in vivo. Dig Dis Sci. 2000;45:763-772. [PubMed] [DOI] |
| 8. | Tan XD, Chang H, Qu XW, Caplan M, Gonzalez-Crussi F, Hsueh W. Platelet-activating factor increases mucosal permeability in rat intestine via tyrosine phosphorylation of E-cadherin. Br J Pharmacol. 2000;129:1522-1529. [PubMed] [DOI] |
| 9. | Ewer AK, Al-Salti W, Coney AM, Marshall JM, Ramani P, Booth IW. The role of platelet activating factor in a neonatal piglet model of necrotising enterocolitis. Gut. 2004;53:207-213. [PubMed] [DOI] |
| 10. | 王 丽杰, 刘 春英, 许 玲芬, 高 红, 姜 卫国, 孙 梅. 血小板活化因子受体拮抗剂对幼年大鼠肠黏膜上皮细胞间紧密连接蛋白的影响. 世界华人消化杂志. 2006;14:392-397. [DOI] |
| 11. | Renes IB, Verburg M, Van Nispen DJ, Büller HA, Dekker J, Einerhand AW. Distinct epithelial responses in experimental colitis: implications for ion uptake and mucosal protection. Am J Physiol Gastrointest Liver Physiol. 2002;283:G169-G179. [PubMed] [DOI] |
| 15. | Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993;123:1777-1788. [PubMed] [DOI] |
| 16. | Hirase T, Staddon JM, Saitou M, Ando-Akatsuka Y, Itoh M, Furuse M, Fujimoto K, Tsukita S, Rubin LL. Occludin as a possible determinant of tight junction permeability in endothelial cells. J Cell Sci. 1997;110:1603-1613. [PubMed] |
| 17. | Saitou M, Ando-Akatsuka Y, Itoh M, Furuse M, Inazawa J, Fujimoto K, Tsukita S. Mammalian occludin in epithelial cells: its expression and subcellular distribution. Eur J Cell Biol. 1997;73:222-231. [PubMed] |
| 18. | Fujimoto K. Freeze-fracture replica electron microscopy combined with SDS digestion for cytochemical labeling of integral membrane proteins. Application to the immunogold labeling of intercellular junctional complexes. J Cell Sci. 1995;108:3443-3449. [PubMed] |
| 19. | Furuse M, Fujita K, Hiiragi T, Fujimoto K, Tsukita S. Claudin-1 and -2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J Cell Biol. 1998;141:1539-1550. [PubMed] [DOI] |
| 20. | Citi S, Sabanay H, Jakes R, Geiger B, Kendrick-Jones J. Cingulin, a new peripheral component of tight junctions. Nature. 1988;333:272-276. [PubMed] [DOI] |
| 21. | Keon BH, Schäfer S, Kuhn C, Grund C, Franke WW. Symplekin, a novel type of tight junction plaque protein. J Cell Biol. 1996;134:1003-1018. [PubMed] [DOI] |
| 22. | Zhong Y, Saitoh T, Minase T, Sawada N, Enomoto K, Mori M. Monoclonal antibody 7H6 reacts with a novel tight junction-associated protein distinct from ZO-1, cingulin and ZO-2. J Cell Biol. 1993;120:477-483. [PubMed] [DOI] |
| 23. | Weber E, Berta G, Tousson A, St John P, Green MW, Gopalokrishnan U, Jilling T, Sorscher EJ, Elton TS, Abrahamson DR. Expression and polarized targeting of a rab3 isoform in epithelial cells. J Cell Biol. 1994;125:583-594. [PubMed] [DOI] |
| 24. | Yamamoto T, Harada N, Kano K, Taya S, Canaani E, Matsuura Y, Mizoguchi A, Ide C, Kaibuchi K. The Ras target AF-6 interacts with ZO-1 and serves as a peripheral component of tight junctions in epithelial cells. J Cell Biol. 1997;139:785-795. [PubMed] [DOI] |
| 25. | Izumi Y, Hirose T, Tamai Y, Hirai S, Nagashima Y, Fujimoto T, Tabuse Y, Kemphues KJ, Ohno S. An atypical PKC directly associates and colocalizes at the epithelial tight junction with ASIP, a mammalian homologue of Caenorhabditis elegans polarity protein PAR-3. J Cell Biol. 1998;143:95-106. [PubMed] [DOI] |
| 26. | Rabinovici R, Yue TL, Farhat M, Smith EF, Esser KM, Slivjak M, Feuerstein G. Platelet activating factor (PAF) and tumor necrosis factor-alpha (TNF alpha) interactions in endotoxemic shock: studies with BN 50739, a novel PAF antagonist. J Pharmacol Exp Ther. 1990;255:256-263. [PubMed] |
| 27. | Artursson P, Palm K, Luthman K. Caco-2 monolayers in experimental and theoretical predictions of drug transport. Adv Drug Deliv Rev. 2001;46:27-43. [PubMed] [DOI] |