修回日期: 2008-09-12
接受日期: 2008-09-17
在线出版日期: 2008-10-28
目的: 探讨黏膜地址素细胞黏附分子-1(MAdCAM-1)在三硝基苯磺酸(TNBS)诱导大鼠结肠炎发病过程中的作用; 研究清肠栓是否通过抑制MAdCAM-1在结肠黏膜的表达, 而发挥其抗炎愈疡作用.
方法: 用TNBS制备实验性大鼠结肠炎模型, 大鼠随机分为清肠栓高剂量组、清肠栓低剂量组、柳氮磺胺吡啶组(SASP)、模型Ⅰ组、模型Ⅱ组及正常组. 模型Ⅰ组造模3 d时, 其余5组在给药7 d时处死大鼠. 取大鼠结肠病变部位标本, 进行组织病理学评价, 用双抗体夹心酶联免疫吸附法检测结肠组织中LTB4和TNF-α水平, 免疫组化染色法和Western blot分析法检测结肠黏膜MAdCAM-1蛋白表达.
结果: 造模3 d时, 模型Ⅰ组大鼠结肠黏膜出现明显组织损伤; 经过7 d处理后, 清肠栓组大鼠黏膜组织损伤减轻. 模型组结肠黏膜LTB4和TNF-α水平比正常组上升(436.38±66.56, 396.81±69.43 vs 203.76±42.84; 394.78±61.53, 413.43±47.39 vs 233.84±55.24, 均P<0.01); 与模型Ⅱ组比较, 各治疗组LTB4和TNF-α水平均下降, 尤以清肠栓高剂量组下降明显(275.74±36.35, 282.72±47.94, P<0.01). 正常组大鼠结肠黏膜固有层MAdCAM-1可见少量表达, 模型Ⅰ组大鼠结肠黏膜阳性染色细胞数明显增多; 与模型Ⅱ组比较, 清肠栓高剂量组、低剂量组和SASP组MAdCAM-1表达均明显下调, 尤其在清肠栓高剂量组下调更为显著.
结论: 清肠栓通过抑制结肠黏膜促炎症因子LTB4和TNF-α释放, 以及下调MAdCAM-1表达, 而发挥其抗炎愈疡作用.
引文著录: 张亚利, 唐志鹏, 李凯, 戴彦成, 何新颖. 清肠栓对三硝基苯磺酸诱导结肠炎大鼠结肠黏膜地址素细胞黏附分子-1表达的影响. 世界华人消化杂志 2008; 16(30): 3381-3386
Revised: September 12, 2008
Accepted: September 17, 2008
Published online: October 28, 2008
AIM: To investigate the role of mucosal addressin cell adhesion molecule-1 (MAdCAM-1) in the pathogenesis of trinitrobenzene sulfonic acid (TNBS) induced rat colitis and to investigate the mechanisms underlying the anti-inflammatory action of Qingchang Supporistory (QCS).
METHODS: The TNBS induced rat colitis model was established. Rats were randomly divided into QCS high-dose group, QCS low-dose group, salicylazosulfapyridine (SASP) group, model Ⅰgroup, model Ⅱ group and normal group. Rats in model Ⅰ group were killed at day 3 while other rats were killed after 7 days' treatment. Histopathological assessment of the colonic mucosa was performed. LTB4 and TNF-α in the colonic mucosa were determined using sandwich enzyme-linked immunosorbent assay (ELISA). Expression of MAdCAM-1 was determined using immunohistochemistry staining and Western blot.
RESULTS: Three days after TNBS administration, colonic mucosal injury occurred in model Ⅰ group while colonic mucosal injury was attenuated in QCS group after 7 days' treatment of QCS. Compared with normal group, the levels of LTB4 and TNF-α in colonic mucosal were raised in the model group (436.38 ± 66.56, 396.81 ± 69.43 vs 203.76 ± 42.84; 394.78 ± 61.53, 413.43 ± 47.39 vs 233.84 ± 55.24, P < 0.01). Compared with model II group, the colonic mucosa levels of LTB4 and TNF-α of all treatment groups were markedly decreased, especially in QCS high-dose group (275.74 ± 36.35, 282.72 ± 47.94, both P < 0.01). MAdCAM-1 was constitutively expressed on the lamina propria of normal colonic mucosa and the amount of positive staining cells dramatically were enhanced in model Ⅰ group. Compared with model Ⅱ group, the expression of MAdCAM-1 was significantly down-regulated in QCS high-dose group, QCS low-dose group and SASP group.
CONCLUSION: QCS performs significant anti-inflammatory action likely through inhibiting colonic mucosal LTB4 and TNF-α production as well as down-regulating MAdCAM-1 expression.
- Citation: Zhang YL, Tang ZP, Li K, Dai YC, He XY. Effect of Qingchang Supporsitory on expression of MAdCAM-1 in the colonic mucosa of rats with TNBS-induced colitis. Shijie Huaren Xiaohua Zazhi 2008; 16(30): 3381-3386
- URL: https://www.wjgnet.com/1009-3079/full/v16/i30/3381.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v16.i30.3381
溃疡性结肠炎(ulcerative colitis, UC)是以腹泻、黏液脓血便、腹痛及里急后重等为主要症状, 以结肠黏膜慢性炎症和溃疡形成为病理特点的一种消化系疾病. UC的病因及发病机制尚未完全明确, 已知肠道黏膜免疫系统异常反应所导致的炎症反应在UC发病中起重要作用. 黏膜地址素细胞黏附分子-1(mucosal addressin cell adhesion molecule-1, MAdCAM-1)是一种表达于肠道及其相关淋巴组织血管内皮细胞表面的免疫球蛋白超家族内皮细胞黏附分子[1-2], 研究表明MAdCAM-1表达增加与UC的发生、发展密切相关[3-5]. 过去研究表明, 中药制剂清肠栓有促进炎症吸收和溃疡愈合的作用, 并能抑制多种炎症介质、细胞因子等致病因子[6-8]. 本文旨在研究清肠栓对三硝基苯磺酸(TNBS)诱导结肠炎大鼠结肠黏膜MAdCAM-1表达的影响, 探讨是否清肠栓通过抑制结肠黏膜MAdCAM-1表达而发挥其抗炎愈疡作用.
♂清洁级健康Wistar大鼠60只, 体质量190-210 g(购自上海斯莱克实验动物有限责任公司). 随机分为: 正常组、模型Ⅰ组、模型Ⅱ组、柳氮磺胺吡啶(SASP)组、清肠栓高剂量组和清肠栓低剂量组, 每组10只. 清肠栓由马齿苋、青黛、参三七、五倍子等组成, 上海中医药大学附属龙华医院自制药品; 柳氮磺吡啶栓(SASP)由山西三九同达药业有限公司生产. 50 g/L 2, 4, 6 TNBS购自美国Sigma公司; 山羊抗大鼠MAdCAM-1多克隆抗体购自美国Santa Cruz公司; 辣根酶标记驴抗山羊二抗购自康成生物工程有限公司; SP免疫组化试剂盒及DAB显色试剂盒(20×)购自北京中杉金桥生物技术有限公司; 大鼠白三烯B4(LTB4)定量EIA试剂盒、大鼠肿瘤坏死因子-α(TNF-α)定量EIA试剂盒均购自上海森雄科技实业有限公司. 其余试剂均为分析纯.
1.2.1 动物模型的制作: 除正常组之外, 其他组大鼠参考文献制备TNBS诱导结肠炎大鼠模型[9]. 造模前将大鼠禁食不禁水24 h后, ip氯胺酮2 mL/kg麻醉大鼠, 用大鼠直灌胃针从肛门插入肠道深约8 cm, 向每只大鼠结肠内注入100 mg/kg浓度TNBS原液0.4 mL+500 mL/L乙醇溶液0.25 mL, 然后注入约0.3 mL空气, 并用夹子夹住大鼠肛门约2 h. 造模后大鼠归笼, 自然清醒, 自由饮食.
1.2.2 给药方法: 于造模后3 d开始, 各组大鼠经肛门给药. 清肠栓高剂量组: 剂量为672 mg/(kg·d), 相当于成人常用量的20倍; 清肠栓低剂量组: 剂量为336 mg/(kg·d), 相当于成人常用量的10倍; SASP组: 剂量为200 mg/(kg·d), 相当于成人常用量的10倍; 正常组和模型对照组大鼠给予等体积(1.5 mL/kg)的生理盐水. 用大鼠直灌胃针从肛门插入肠道深约8 cm, 给药1次/d, 连续给药7 d.
1.2.3 标本采集: 模型Ⅰ组于3 d, 其余各组于给药7 d(即造模处理10 d)后处死大鼠, 开腹分离结肠, 沿肠系膜纵轴剪开肠腔, 剪取距肛门8-12 cm结肠段, 纵向剖开, 冰生理盐水冲洗干净后平展于过滤纸上, 一部分结肠组织经40 g/L的中性甲醛溶液固定后, 常规脱水、浸蜡、透明、包埋, 4 µm厚度切片, 分别行常规HE染色和免疫组化染色. 其余部分结肠组织迅速冷冻于液氮中, 后转至-80℃冰箱保存. 留待行ELISA试剂盒检测LTB4和TNF-α的含量及进行Western blot检测.
1.2.4 结肠组织病理形态学观察: 切片HE染色后以Olympus显微镜观察并拍照.
1.2.5 结肠组织LTB4和TNF-α水平的测定: 采用LTB4和TNF-α定量EIA试剂盒检测.
1.2.6 免疫组化检测MAdCAM-1蛋白的表达: 采用SP法染色, 操作过程按照试剂盒说明书进行, 山羊抗大鼠MAdCAM-1多克隆抗体稀释倍数为1:100, DAB显色, 苏木素复染, 中性树胶封片, 光镜下观察.
1.2.7 Western blot方法检测清肠栓对结肠组织MAdCAM-1表达的影响: 提取组织总蛋白, 考马斯亮蓝法测定其含量. 取50 µg总蛋白经10%分离胶, 5%堆积胶凝胶电泳分离并转移至硝酸纤维素膜上. 用含50 g/L脱脂奶粉的TTBS室温封闭1 h; 加1:100稀释的山羊抗大鼠MAdCAM-1多克隆抗体, 4℃, 摇床过夜; 将膜以TTBS漂洗5 min×3次, 加入1:2500稀释的HRP偶联的驴抗山羊二抗, 室温孵育1 h; 将膜以TTBS漂洗5 min×2次, 再次漂洗15 min×1次, 以除去未结合的2抗. ECL显色, 去离子水漂洗. 应用复日FR-200生物电泳图象分析系统分析照相记录结果.
统计学处理 所有计量资料的数据均以mean±SD表示, 各组数据进行正态检验及方差齐性检验, 采用SPSS11.5统计软件进行单因素方差分析(analytic of variance, ANOVA)和LSD-t(least significant difference-t)检验进行组间比较, P<0.05为具有统计学意义.
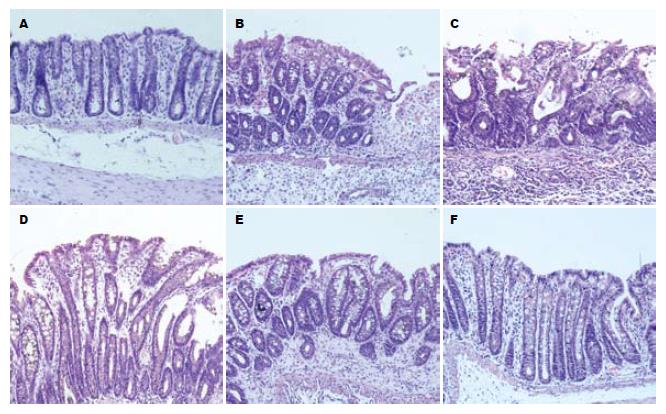
正常组大鼠结肠黏膜上皮完整、连续, 细胞与腺体排列规则, 黏膜、固有层内血管和纤维间质正常, 肌层无异常; 模型Ⅰ组大鼠结肠黏膜出现广泛坏死、糜烂、腺体结构紊乱, 可见溃疡及隐窝脓肿形成, 个别溃疡深达黏膜下层甚至肌层, 炎症细胞浸润全层, 并可见到淋巴滤泡及巨噬细胞. 模型Ⅱ组仍有黏膜层糜烂, 黏膜及黏膜下层炎细胞浸润较前有所好转, 溃疡底部已见肉芽组织增生, 部分糜烂黏膜表面已有上皮移行修复迹象. 清肠栓低剂量组和SASP组可见程度不同的黏膜上皮移行修复, 肉芽组织增生, 固有层中腺体呈管状增粗, 可见散在淋巴细胞及巨噬细胞. 清肠栓高剂量组黏膜下层和肌层轻度水肿, 炎症细胞减少, 并见肉芽组织参与修补, 多数表面已出现上皮修复, 黏膜腺体恢复接近正常(图1).
与正常组比较, 模型Ⅰ组及模型Ⅱ组大鼠病变结肠组织LTB4和TNF-α水平均显著升高(P<0.01); 清肠栓高剂量组较模型Ⅱ组明显降低(P<0.01). 清肠栓低剂量组和SASP组结肠组织LTB4和TNF-α水平较模型Ⅱ组明显降低(P<0.05), 清肠栓低剂量组与SASP组比较, 两者差异无统计学意义(表1).
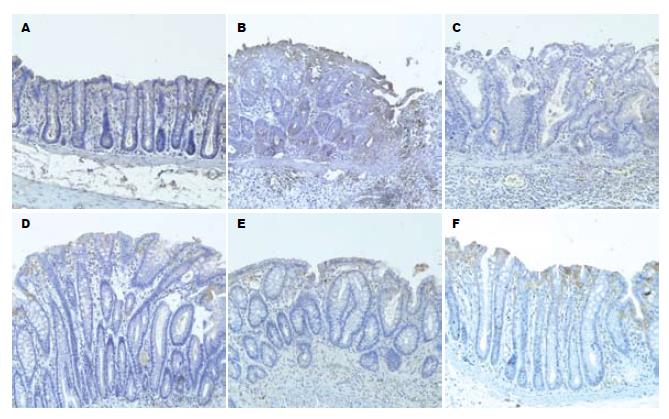
免疫组化染色显示, 结肠黏膜MAdCAM-1阳性表达的细胞为棕黄色或棕褐色, 并呈颗粒状. 正常组大鼠结肠黏膜固有层可见少量表达; 模型Ⅰ组及模型Ⅱ组大鼠结肠黏膜阳性染色细胞数较正常组明显增多, 尤其以模型Ⅰ组增高更为显著; 清肠栓高剂量组、低剂量组和SASP组MAdCAM-1表达均低于模型Ⅱ组, 其中清肠栓高剂量组低于清肠栓低剂量组、SASP组(图2).
分析结肠组织MAdCAM-1蛋白条带平均密度值发现, 模型Ⅰ组及模型Ⅱ组大鼠结肠黏膜MAdCAM-1表达水平升高, 尤以模型Ⅰ组升高更为明显, 与正常组比较有显著性差异(P<0.01); 清肠栓高剂量组、低剂量组和SASP组MAdCAM-1表达与模型Ⅱ组比较显著降低, 以清肠栓高剂量组降低最为显著(P<0.01); 清肠栓低剂量组和SASP组MAdCAM-1表达水平高于清肠栓高剂量组(P<0.01), 清肠栓低剂量组和SASP组间比较无统计学意义(图3).
在我国, 近年来由于生活水平的不断提高, 饮食结构、生活习惯的改变, 加之检查诊断技术的提高, UC发病率亦呈逐年上升趋势. UC病因和发病机制复杂, 涉及遗传、环境、感染等诸多因素, 而免疫因素在UC发病中起着至关重要的作用. UC的发病不仅涉及系统免疫功能紊乱, 还存在着肠道黏膜局部免疫功能的异常. 目前临床治疗尚不理想.清肠栓由马齿苋、五倍子、青黛、参三七等药味组成, 具有清热解毒化湿, 凉血化瘀止血, 祛腐生新愈疡之功. 临床实践证明其治疗UC疗效较好, 是中药治疗UC的有效方剂.
炎症因子参与免疫反应和炎症过程, 特别是LTB4、TNF-α在UC的发病中颇受重视. LTB4与炎症、变态反应、血小板聚集等反应密切相关, 对胃肠道的病理生理有很大影响, 可以引起黏膜损伤[10]. 尽管UC的病因尚未完全明确, LTB4被认为是引起UC炎症反应的重要介质, 在UC炎症形成过程中发挥重要作用[11]. LTB4促进白细胞向炎症部位游走、聚集, 黏附于血管内皮细胞, 脱颗粒, 释放氧自由基及溶酶体酶, 还可增加血管壁通透性, 导致炎症反应[12-13]. TNF-α是介导UC发病的促炎症细胞因子之一, 在肠道中亦能介导肠黏膜的损伤[14-15]. TNF-α可调节中性粒细胞和巨噬细胞的增生、成熟和活化, 并促进其黏附、游走和脱颗粒, 增强他们的吞噬杀伤功能, 促进他们释放炎症蛋白和炎症介质, 直接参与肠黏膜的损伤.
MAdCAM-1属于免疫球蛋白超家族成员,为60 kDa的单链跨膜糖蛋白. 分胞外、胞内、跨膜区三部分. 正常情况下, MAdCAM-1选择性地表达于派伊尔淋巴结(Peyer's patch)、肠系膜淋巴结的高内皮静脉(high endothelial venules), 以及小肠和结肠黏膜固有层中的扁平血管内皮细胞表面, 极少表达于肠道外组织.临床和动物实验研究证实, UC与MAdCAM-1的异常表达有关. UC患者的炎症结肠部位血管内皮细胞MAdCAM-1表达上调[3,16]; MAdCAM-1在UC动物模型表达亦显著增加[17-19]. 在DSS诱导慢性结肠炎中, 通过阻断淋巴细胞毒素β受体活性, 可以降低MAdCAM-1表达进而减少淋巴细胞迁移, 使炎症得到缓解[20]. 此外, 减少肠内刺激包括静脉营养时缺乏肠道喂养以及禁食等能够显著降低MAdCAM-1表达水平[21-22]. 细胞因子参与诱导MAdCAM-1的表达. 例如在TNF-α诱导下, MAdCAM-1在肠黏膜内皮细胞的表达显著上调[23]. 研究发现, 磷脂酰肌醇3-激酶(phosphatidylinositol 3-kinase, PI3-K)介导的Akt活化作用参与了TNF-α和脂多糖(lipopolysaccharide, LPS)诱导血管内皮细胞MAdCAM-1表达过程[24]. MAdCAM-1独特表达于肠道黏膜, 因此调控MAdCAM-1表达有可能成为UC的器官特异性有效治疗策略[25]. Kato et al[26]应用抗MAdCAM-1抗体治疗DSS诱导小鼠实验性结肠炎, 使用免疫组化染色方法检测淋巴细胞及其亚群和细胞黏附分子的表达, 结果发现抗MAdCAM-1抗体使结肠黏膜β7阳性淋巴细胞浸润数量减少, 明显减轻结肠黏膜的损伤和炎症. Goto et al[27]报道, 用MAdCAM-1反义寡核苷酸治疗TNBS诱导小鼠实验性结肠炎, 表明MAdCAM-1反义寡核苷酸能够下调结肠组织MAdCAM-1蛋白和mRNA表达, 减少炎症部位淋巴细胞上α4β7的数量, 显著减轻结肠炎的症状和抑制其组织病理学进展.
本实验结果表明正常组大鼠结肠黏膜固有层MAdCAM-1阳性细胞有少量表达, 模型Ⅰ组及模型Ⅱ组大鼠结肠黏膜阳性染色细胞数较正常组明显增多; 清肠栓高剂量组、低剂量组和SASP组MAdCAM-1表达明显低于模型Ⅱ组, 其中清肠栓高剂量组低于清肠栓低剂量组和SASP组. 采用Western blot方法定量检测清肠栓对TNBS诱导结肠炎大鼠结肠黏膜MAdCAM-1表达的影响, 结果显示模型Ⅰ组、模型Ⅱ组大鼠结肠黏膜MAdCAM-1的表达水平均较正常组显著升高, 提示其在UC的发病机制中可能起重要作用. LTB4、TNF-α参与诱导MAdCAM-1表达. 我们发现模型Ⅰ组及Ⅱ组结肠组织LTB4、TNF-α水平较正常组显著上升, 可能LTB4、TNF-α介导TNBS诱导结肠炎大鼠结肠黏膜MAdCAM-1表达上调, 后者通过介导循环中的淋巴细胞进入炎症部位而导致组织损伤. 经治疗后清肠栓高剂量组、清肠栓低剂量组与SASP组MAdCAM-1的表达水平较模型Ⅱ组显著降低, 不仅如此, 较正常水平也明显下降, 尤其以清肠栓高剂量组降低最为显著; 治疗7 d后随着症状的改善, 无论清肠栓组或是SASP组, 其结肠组织LTB4、TNF-α水平皆呈下降趋势. 提示清肠栓可能通过抑制大鼠结肠黏膜LTB4、TNF-α释放, 进而抑制结肠黏膜MAdCAM-1表达, 而发挥其消炎愈疡作用.
清肠栓是在参阅古代医学文献, 在锡类散、青黛散治疗黏膜溃疡理论的基础上, 经过多年临床实践优化筛选出来的有效方剂, 在临床应用中取得很好疗效, 能止血止痛止泻, 持续应用能有效防止该病复发, 是中药治疗该病的有效方剂. 本实验对清肠栓治疗UC的疗效及机制进行了初步研究, 为中医药治疗UC提供了新的思路和方法.
UC是临床常见难治性疾病, 目前西医治疗存在疗效欠佳、复发率高、副作用大和价格昂贵问题. 中医药治疗本病具有优势. 中药清肠栓由马齿苋、五倍子、青黛、参三七等药味组成, 具有清热解毒化湿, 凉血化瘀止血, 祛腐生新愈疡之功, 在临床应用中取得很好疗效,持续应用能有效防止溃疡性结肠炎复发, 是中药治疗该病的有效方剂.
杜群, 副研究员, 广州中医药大学脾胃研究所药理室.
先前研究表明清肠栓可以减轻氧化损伤; 抑制结肠组织促炎细胞因子表达, 促进抑炎细胞因子表达; 调节凋亡相关基因表达, 减少结肠上皮细胞凋亡,并且诱导固有层淋巴细胞凋亡; 促进结肠黏膜细胞增殖、增加杯状细胞的数量和分泌黏液的含量.
本文采用免疫组化和Western blot法检测MAdCAM-1在各组的表达, 采用酶联免疫法检测各组LTB4和TNF-α的含量. 结果提示清肠栓可能通过抑制结肠黏膜促炎症因子LTB4和TNF-α释放, 以及下调MAdCAM-1表达, 发挥其抗炎愈疡作用.
本实验对清肠栓治疗UC的疗效及作用机制进行了初步研究, 为其临床应用和深化研究提供了依据.
黏膜地址素细胞黏附分子-1 (MAdCAM-1): 一种表达于肠道及其相关淋巴组织血管内皮细胞表面的免疫球蛋白超家族内皮细胞黏附分子.
本文研究目的明确, 研究方法先进, 结果可靠, 讨论适当, 有较好的学术价值.
编辑: 李军亮 电编:吴鹏朕
| 1. | Briskin MJ, McEvoy LM, Butcher EC. MAdCAM-1 has homology to immunoglobulin and mucin-like adhesion receptors and to IgA1. Nature. 1993;363:461-464. [PubMed] [DOI] |
| 2. | Tan K, Casasnovas JM, Liu JH, Briskin MJ, Springer TA, Wang JH. The structure of immunoglobulin superfamily domains 1 and 2 of MAdCAM-1 reveals novel features important for integrin recognition. Structure. 1998;6:793-801. [PubMed] [DOI] |
| 3. | Arihiro S, Ohtani H, Suzuki M, Murata M, Ejima C, Oki M, Kinouchi Y, Fukushima K, Sasaki I, Nakamura S. Differential expression of mucosal addressin cell adhesion molecule-1 (MAdCAM-1) in ulcerative colitis and Crohn's disease. Pathol Int. 2002;52:367-374. [PubMed] [DOI] |
| 4. | Briskin M, Winsor-Hines D, Shyjan A, Cochran N, Bloom S, Wilson J, McEvoy LM, Butcher EC, Kassam N, Mackay CR. Human mucosal addressin cell adhesion molecule-1 is preferentially expressed in intestinal tract and associated lymphoid tissue. Am J Pathol. 1997;151:97-110. [PubMed] |
| 5. | van Assche G, Rutgeerts P. Antiadhesion molecule therapy in inflammatory bowel disease. Inflamm Bowel Dis. 2002;8:291-300. [PubMed] [DOI] |
| 6. | 郝 微微, 马 贵同, 张 晓峰, 唐 志鹏, 龚 雨萍, 柳 文, 朱 凌宇. 中药清肠栓对溃疡性结肠炎大鼠白细胞介素4及10 mRNA 表达的影响. 中国中西医结合消化杂志. 2007;15:177-180. |
| 7. | 张 涛, 谢 建群. 清肠栓对大鼠溃疡性结肠炎结肠黏膜固有层淋巴细胞凋亡及血清IL-1β与IL-13的影响. 上海中医药大学 学报. 2006;20:37-40. |
| 8. | 王 臻楠, 唐 志鹏, 马 贵同, 张 亚历. 清肠栓调节三硝基苯磺酸诱导结肠炎大鼠结肠黏膜细胞增殖的影响. 中国中西医 结合消化杂志. 2006;14:383-386. |
| 9. | Morris GP, Beck PL, Herridge MS, Depew WT, Szewczuk MR, Wallace JL. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989;96:795-803. [PubMed] |
| 10. | Shimizu T, Suzuki M, Fujimura J, Hisada K, Yoshikazu O, Obinata K, Yamashiro Y. The relationship between the concentration of dextran sodium sulfate and the degree of induced experimental colitis in weanling rats. J Pediatr Gastroenterol Nutr. 2003;37:481-486. [PubMed] [DOI] |
| 11. | Forbes A. Alternative immunomodulators. Eur J Gastroenterol Hepatol. 2003;15:245-248. [PubMed] [DOI] |
| 12. | Krueger KJ, McClain CJ, McClave SA, Dryden GW. Nutritional supplements and alternative medicine. Curr Opin Gastroenterol. 2004;20:130-138. [PubMed] [DOI] |
| 13. | Everest PH, Cole AT, Hawkey CJ, Knutton S, Goossens H, Butzler JP, Ketley JM, Williams PH. Roles of leukotriene B4, prostaglandin E2, and cyclic AMP in Campylobacter jejuni-induced intestinal fluid secretion. Infect Immun. 1993;61:4885-4887. [PubMed] |
| 14. | Olsen T, Goll R, Cui G, Husebekk A, Vonen B, Birketvedt GS, Florholmen J. Tissue levels of tumor necrosis factor-alpha correlates with grade of inflammation in untreated ulcerative colitis. Scand J Gastroenterol. 2007;42:1312-1320. [PubMed] |
| 15. | Stucchi A, Reed K, O'Brien M, Cerda S, Andrews C, Gower A, Bushell K, Amar S, Leeman S, Becker J. A new transcription factor that regulates TNF-alpha gene expression, LITAF, is increased in intestinal tissues from patients with CD and UC. Inflamm Bowel Dis. 2006;12:581-587. [PubMed] [DOI] |
| 16. | Souza HS, Elia CC, Spencer J, MacDonald TT. Expression of lymphocyte-endothelial receptor-ligand pairs, alpha4beta7/MAdCAM-1 and OX40/OX40 ligand in the colon and jejunum of patients with inflammatory bowel disease. Gut. 1999;45:856-863. [PubMed] |
| 17. | Viney JL, Jones S, Chiu HH, Lagrimas B, Renz ME, Presta LG, Jackson D, Hillan KJ, Lew S, Fong S. Mucosal addressin cell adhesion molecule-1: a structural and functional analysis demarcates the integrin binding motif. J Immunol. 1996;157:2488-2497. [PubMed] |
| 18. | Kawachi S, Jennings S, Panes J, Cockrell A, Laroux FS, Gray L, Perry M, van der Heyde H, Balish E, Granger DN. Cytokine and endothelial cell adhesion molecule expression in interleukin-10-deficient mice. Am J Physiol Gastrointest Liver Physiol. 2000;278:G734-G743. [PubMed] |
| 19. | Shigematsu T, Specian RD, Wolf RE, Grisham MB, Granger DN. MAdCAM mediates lymphocyte-endothelial cell adhesion in a murine model of chronic colitis. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1309-G1315. [PubMed] |
| 20. | Stopfer P, Obermeier F, Dunger N, Falk W, Farkas S, Janotta M, Möller A, Männel DN, Hehlgans T. Blocking lymphotoxin-beta receptor activation diminishes inflammation via reduced mucosal addressin cell adhesion molecule-1 (MAdCAM-1) expression and leucocyte margination in chronic DSS-induced colitis. Clin Exp Immunol. 2004;136:21-29. [PubMed] [DOI] |
| 21. | Hermsen JL, Gomez FE, Maeshima Y, Sano Y, Kang W, Kudsk KA. Decreased enteral stimulation alters mucosal immune chemokines. JPEN J Parenter Enteral Nutr. 2008;32:36-44. [PubMed] [DOI] |
| 22. | Gomez FE, Lan J, Kang W, Ueno C, Kudsk KA. Parenteral nutrition and fasting reduces mucosal addressin cellular adhesion molecule-1 (MAdCAM-1) mRNA in Peyer's patches of mice. JPEN J Parenter Enteral Nutr. 2007;31:47-52. [PubMed] [DOI] |
| 23. | Watanabe C, Miura S, Hokari R, Teramoto K, Ogino T, Komoto S, Hara Y, Koseki S, Tsuzuki Y, Nagata H. Spatial heterogeneity of TNF-alpha-induced T cell migration to colonic mucosa is mediated by MAdCAM-1 and VCAM-1. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1379-G1387. [PubMed] |
| 24. | Ogawa H, Binion DG, Heidemann J, Theriot M, Fisher PJ, Johnson NA, Otterson MF, Rafiee P. Mechanisms of MAdCAM-1 gene expression in human intestinal microvascular endothelial cells. Am J Physiol Cell Physiol. 2005;288:C272-C281. [PubMed] [DOI] |
| 25. | Van Assche G, Rutgeerts P. Physiological basis for novel drug therapies used to treat the inflammatory bowel diseases. I. Immunology and therapeutic potential of antiadhesion molecule therapy in inflammatory bowel disease. Am J Physiol Gastrointest Liver Physiol. 2005;288:G169-G174. [PubMed] [DOI] |
| 26. | Kato S, Hokari R, Matsuzaki K, Iwai A, Kawaguchi A, Nagao S, Miyahara T, Itoh K, Ishii H, Miura S. Amelioration of murine experimental colitis by inhibition of mucosal addressin cell adhesion molecule-1. J Pharmacol Exp Ther. 2000;295:183-189. [PubMed] |
| 27. | Goto A, Arimura Y, Shinomura Y, Imai K, Hinoda Y. Antisense therapy of MAdCAM-1 for trinitrobenzenesulfonic acid- induced murine colitis. Inflamm Bowel Dis. 2006;12:758-765. [PubMed] [DOI] |