修回日期: 2007-12-25
接受日期: 2008-01-15
在线出版日期: 2008-01-28
目的: 探讨非酒精性脂肪肝(NAFLD)患者糖代谢异常与血清超敏C反应蛋白(hsCRP)的相关性.
方法: 将191例NAFLD患者按口服葡萄糖耐量试验(OGTT)分为糖代谢正常组和异常组, 检测患者血清hsCRP.
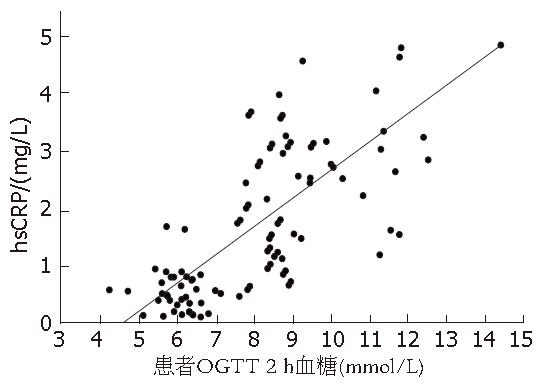
结果: NAFLD患者糖代谢异常组血清hsCRP浓度较糖代谢正常组明显增高(4.01±1.45 vs 0.96±0.41, P<0.01), 血清hsCRP浓度与2 h血糖呈正相关(r = 0.74, P<0.01).
结论: NAFLD患者糖代谢异常与血清hsCRP密切相关.
引文著录: 张炜, 范钰, 朱丽群, 程兆明. 非酒精性脂肪肝糖代谢异常与超敏C反应蛋白的关系. 世界华人消化杂志 2008; 16(3): 319-321
Revised: December 25, 2007
Accepted: January 15, 2008
Published online: January 28, 2008
AIM: To study the relationship between abnormal glycometabolism and ultra-sensitive C-reactive protein (hsCRP) in non-alcoholic fatty liver (NAFLD).
METHODS: One hundred and ninety-one NAFLD patients were divided into normal glucose metabolism group and abnormal glucose metabolism group according to the oral glucose tolerance test (OGTT). Their serum hsCRP levels were measured.
RESULTS: The serum hsCRP levels in the abnormal glucose metabolism group were higher than those in the normal glucose metabolism group(4.01 ± 1.45 vs 0.96 ± 0.41, P < 0.01), which were positively correlated with the glucose tolerance (r = 0.74, P < 0.01).
CONCLUSION: Serum hsCRP levels are related with abnormal glycometabolism in NAFLD patients.
- Citation: Zhang W, Fan Y, Zhu LQ, Cheng ZM. Relationship between abnormal glycometabolism and ultra-sensitive C-reactive protein in non-alcoholic fatty liver. Shijie Huaren Xiaohua Zazhi 2008; 16(3): 319-321
- URL: https://www.wjgnet.com/1009-3079/full/v16/i3/319.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v16.i3.319
非酒精性脂肪肝(NAFLD)指病变主要在肝小叶, 以肝细胞脂肪变性和脂肪沉积为病理特征, 但无过量饮酒史的临床病症, 成年人发病率17%-33%[1]. 胰岛素抵抗是发生发展的基础, 随着肝脏疾病的慢性进展, 肝细胞反复受损伤, 肝纤维化程度加重, 同时, 糖代谢异常及胰岛素抵抗的发生率逐渐增高[2]. 超敏C反应蛋白(hsCRP)是一种敏感、非特异性的炎症标志物, 近年来已被公认为是糖代谢异常的独立危险因素[3-4]. 本文通过不同糖耐量水平的NAFLD患者hsCRP比较, 探讨hsCRP与NAFLD患者糖耐量水平的相关性.
2006-06/2007-06在我院就诊的NAFLD患者191例, 均符合2006年中华医学会肝脏病学分会提出的诊断标准[5], 男138例, 女53例. 所有患者否认就诊病前有糖尿病史及糖尿病家族史. 并除去有明确冠心病和心衰者.
所有患者, 进行口服葡萄糖耐量试验(OGTT, 75 g葡萄糖粉), 按OGTT结果, 将NAFLD患者分为糖代谢正常组和异常组, hsCRP采用免疫散射比浊法, 用岛津7200全自动生化分析仪检测肝功能, 以AST≤40 U/L为正常, 40 U/L<AST≤80 U/L为轻度异常, AST>80 U/L为明显异常.
统计学处理 所有数据用SPSS13.0软件处理. hsCRP水平为计量资料用mean±SD表示, 两样本均数的比较用t检验, 肝功能分级为定性资料, 两组的比较用χ2检验, P<0.01有统计学意义.
NAFLD患者中糖代谢正常89例, 占46.6%; 糖代谢异常102例, 占53.4%. 两组NAFLD患者年龄、性别、体质量指数(BMI)和血压水平无明显差异. 糖代谢异常组血清hsCRP浓度较糖代谢正常组明显增高, t = 19.20, P<0.01(表1).
| 分组 | n | 血清hsCRP(mg/L) |
| 糖代谢正常组 | 89 | 0.96±0.41 |
| 糖代谢异常组 | 102 | 4.01±1.45 |
| 正常参考值 | 0.65±0.35 |
2 h血糖相关性 NAFLD患者血清hsCRP与OGTT 2 h血糖呈明显的正相关, r = 0.74, P<0.01(图1).
NAFLD是一种临床病理综合征, 他的范畴从单纯的脂肪变性到非酒精性脂肪性肝炎、纤维化和终末期肝病[6]. 由于目前尚无特异的治疗方案, 其机制的研究对于阻止NAFLD的进展有重要意义[7]. 最近的研究结果表明, 胰岛素抵抗可能参与了其病理生理过程[8]. 随着肝脏疾病的慢性化进展, 糖代谢异常的发生率逐渐增高, 常伴有高血糖和高胰岛素血症. 这种现象与肝脏对血糖摄取、糖原合成及分解功能障碍及高血糖刺激胰岛素分泌, 肝脏摄取、降解胰岛素的作用减弱等因素有关, 也存在有明显的胰岛素抵抗[9]. 本研究结果显示NAFLD患者糖代谢异常的发生率高达53.4%.
在NAFLD发生发展过程中炎症是重要的环节[10]. 本研究结果显示可见NAFLD患者随着糖代谢异常的发生肝功能损伤更加明显. 说明炎症与糖代谢异常密切相关. hsCRP是一项灵敏度较高的炎症反应性指标, 已经被作为公认的心血管疾病预示因子用于预测心血管事件的发生和死亡率[11-12]. 同时hsCRP水平也与糖代谢异常密切相关[13]. Temelkova-Kurktschiev et al[14]和Festa et al[15]研究结果已经证实, CRP与血糖相关的原因与高血糖及胰岛素水平异常等状态导致血管内皮细胞损伤, 引起炎症反应有关. 而脂肪肝为肝脏内过多的脂肪沉积, 且常伴有内脏脂肪增多及胰岛素抵抗, 从发病因素看与此类似[16]. 因此NAFLD患者hsCRP水平可能增高. 本研究结果显示NAFLD患者随着糖代谢异常的发生hsCRP 水平升高. 而且hsCRP水平与OGTT 2 h血糖有明显的正相关.
总之, NAFLD患者血清hsCRP水平反应炎症, 炎症与胰岛素抵抗密切相关, 互为因果. 通过检测血清hsCRP可以判断NAFLD患者肝脏炎症与合并糖代谢异常的情况, 监测病情变化.
hsCRP是一种敏感的炎症标志物, 近年来已被公认为是糖代谢异常的独立危险因素. NAFLD患者随着疾病的进展, 糖代谢异常的发生率逐渐增高, 常伴有高血糖和高胰岛素血症.
黄恒青, 主任医师, 福建省第二人民医院消化内科
通过检测血清 hsCRP可以判断NAFLD患者肝脏炎症与合并糖代谢异常, hsCRP可能成为判断患者病情的新指标.
本文实验设计合理, 论据充分, 具有一定的临床价值.
编辑: 李军亮 电编: 何基才
| 2. | Petrides AS, Schulze-Berge D, Vogt C, Matthews DE, Strohmeyer G. Glucose resistance contributes to diabetes mellitus in cirrhosis. Hepatology. 1993;18:284-291. [PubMed] [DOI] |
| 3. | Leinonen E, Hurt-Camejo E, Wiklund O, Hultén LM, Hiukka A, Taskinen MR. Insulin resistance and adiposity correlate with acute-phase reaction and soluble cell adhesion molecules in type 2 diabetes. Atherosclerosis. 2003;166:387-394. [PubMed] [DOI] |
| 4. | Xu Y, Whitmer K. C-reactive protein and cardiovascular disease in people with diabetes: high-sensitivity CRP testing can help assess risk for future cardiovascular disease events in this population. Am J Nurs. 2006;106:66-72. [PubMed] [DOI] |
| 6. | Grant LM, Lisker-Melman M. Nonalcoholic fatty liver disease. Ann Hepatol. 2004;3:93-99. [PubMed] |
| 7. | Tilg H, Kaser A. Treatment strategies in nonalcoholic fatty liver disease. Nat Clin Pract Gastroenterol Hepatol. 2005;2:148-155. [PubMed] [DOI] |
| 8. | Cortez-Pinto H, Camilo ME. Non-alcoholic fatty liver disease/non-alcoholic steatohepatitis (NAFLD/NASH): diagnosis and clinical course. Best Pract Res Clin Gastroenterol. 2004;18:1089-1104. [PubMed] [DOI] |
| 9. | Marchesini G, Marzocchi R, Agostini F, Bugianesi E. Nonalcoholic fatty liver disease and the metabolic syndrome. Curr Opin Lipidol. 2005;16:421-427. [PubMed] [DOI] |
| 10. | Panasiuk A, Dzieciol J, Panasiuk B, Prokopowicz D. Expression of p53, Bax and Bcl-2 proteins in hepatocytes in non-alcoholic fatty liver disease. World J Gastroenterol. 2006;12:6198-6202. [PubMed] [DOI] |
| 11. | Kovacs A, Green F, Hansson LO, Lundman P, Samnegård A, Boquist S, Ericsson CG, Watkins H, Hamsten A, Tornvall P. A novel common single nucleotide polymorphism in the promoter region of the C-reactive protein gene associated with the plasma concentration of C-reactive protein. Atherosclerosis. 2005;178:193-198. [PubMed] [DOI] |
| 12. | Haffner SM. The metabolic syndrome: inflammation, diabetes mellitus, and cardiovascular disease. Am J Cardiol. 2006;97:3A-11A. [PubMed] [DOI] |
| 13. | Pietri P, Vyssoulis G, Vlachopoulos C, Zervoudaki A, Gialernios T, Aznaouridis K, Stefanadis C. Relationship between low-grade inflammation and arterial stiffness in patients with essential hypertension. J Hypertens. 2006;24:2231-2238. [PubMed] [DOI] |
| 14. | Temelkova-Kurktschiev T, Siegert G, Bergmann S, Henkel E, Koehler C, Jaross W, Hanefeld M. Subclinical inflammation is strongly related to insulin resistance but not to impaired insulin secretion in a high risk population for diabetes. Metabolism. 2002;51:743-749. [PubMed] [DOI] |
| 15. | Festa A, D'Agostino R, Tracy RP, Haffner SM. Elevated levels of acute-phase proteins and plasminogen activator inhibitor-1 predict the development of type 2 diabetes: the insulin resistance atherosclerosis study. Diabetes. 2002;51:1131-1137. [PubMed] [DOI] |
| 16. | Videla LA, Rodrigo R, Araya J, Poniachik J. Insulin resistance and oxidative stress interdependency in non-alcoholic fatty liver disease. Trends Mol Med. 2006;12:555-558. [PubMed] [DOI] |