修回日期: 2008-07-24
接受日期: 2008-08-04
在线出版日期: 2008-09-18
目的: 探讨RASSF1A与CyclinD1在胃癌发生发展中的作用.
方法: 采用RT-PCR检测胃正常组织、腺瘤组织、不典型增生组织各20例及胃腺癌组织40例中RASSF1A及CyclinD1 mRNA的表达, 并Western blot法检测RASSF1A蛋白表达.
结果: 胃腺癌组RASSF1A的表达低于不典型增生、良性腺瘤及正常组织组(37.5% vs 80.0%, 95.0%, 100.0%, 均P<0.05), 而CyclinD1的表达高于不典型增生、良性腺瘤及正常组织组(77.5% vs 25.0%, 10.0%, 5.0%, 均P<0.05). 胃癌组织中,RASSF1A及CyclinD1 mRNA表达均与病理分级有关(χ2 = 4.422, 8.935, 均P<0.05); 两者之间表达呈负相关(r = -0.448, P<0.05); RASSF1A蛋白表达与mRNA表达一致.
结论: RASSF1A与CyclinD1两种蛋白的异常表达在胃癌的发生发展中可能具有协同作用, 二者联合检测能为胃癌的早期临床诊断和治疗提供有利的生物学信息.
引文著录: 蒋鹏程, 马圭, 孟鑫, 祁卫东, 高振军. RASSF1A基因在胃腺癌和癌前病变中的表达及意义. 世界华人消化杂志 2008; 16(26): 2992-2996
Revised: July 24, 2008
Accepted: August 4, 2008
Published online: September 18, 2008
AIM: To investigate the role of RASSF1A and CyclinD1 in the development of gastric cancer.
METHODS: RT-PCR was used to detect the mRNA expression of RASSF1A and CyclinD1 in 20 cases of normal gastric tissues, gastric adenoma and atypical hyperplasia, and 40 cases of gastric adenocarcinoma while Western blot was adopted to detect the protein expression of RASSF1A.
RESULTS: The expression of RASSF1A was lower in gastric adenocarcinoma than in atypical hyperplasia, gastric adenoma and normal gastric tissues (37.5% vs 80.0%, 95.0%, 100.0%, all P < 0.05). However, the expression of CyclinD1 was significantly higher than in atypical hyperplasia, gastric adenoma and normal gastric tissues (77.5% vs 25.0%, 10.0%, 5.0%, all P < 0.05). In gastric cancer tissues, both of the expressions of RASSF1A and CyclinD1 mRNA were associated with pathological grade (χ2 = 4.422, P < 0.05; χ2 = 8.935, P < 0.05); their expressions were negatively correlated(r = -0.448, P < 0.05), while the expressions of RASSF1A protein and mRNA were consistent.
CONCLUSION: The silent expression of RASSF1A and the increased expression of CyclinD1 may play an important role in gastric cancer pathogenesis. Their combined detection can contribute to the earlier diagnosis and treatment of gastric cancer.
- Citation: Jiang PC, Ma G, Meng X, Qi WD, Gao ZJ. Expression of RASSF1A and CyclinD1 in gastric cancer and premalignant lesion and its significance. Shijie Huaren Xiaohua Zazhi 2008; 16(26): 2992-2996
- URL: https://www.wjgnet.com/1009-3079/full/v16/i26/2992.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v16.i26.2992
胃癌是人类常见的恶性肿瘤, 居全球肿瘤发病和癌症死亡率的第二位[1], 胃癌的发生是一个多步骤多因素进行性发展的过程, 癌基因的激活、抑癌基因的抑制, 及其他一些肿瘤相关因子的作用, 使上皮细胞过度增殖而又不能启动凋亡信号时, 则可能逐渐发展为胃癌[2-3]. 探求相关的癌基因、抑癌基因在肿瘤发生发展中的作用, 是明确肿瘤发病机制和防治的关键因素. RASSF1A是新近发现的抑癌基因[4], CyclinD1是细胞周期重要的调控因子[5]. 本课题旨在研究RASSF1A及CyclinD1 mRNA在胃癌及癌前病变中的表达, 探讨RASSF1A基因与胃癌发生发展的关系.
选取2007-01/2007-06江苏大学附属人民医院行手术切除的40例胃癌标本, 患者年龄30-85(平均57.6±13.7)岁, 均经病理确诊, 术前未用任何放、化疗, 手术时收集癌组织和距癌缘5 cm以外的癌旁正常组织, 快速置入液氮罐, 然后转入-80 ℃冰箱储存备用. 取同期胃癌前病变(中重度慢性萎缩性胃炎伴肠上皮化生和不典型增生)的胃镜活检标本20例, 经病理证实均为良性腺瘤, 迅速置于液氮中保存备用. RASSF1A鼠抗人mAb购自eBioscience公司, 内参GAPDH购自KangChen公司, PVD膜购自Bio-Rad公司, ECL发光试剂盒购自GE公司, PCR引物由上海生工合成.
1.2.1 RT-PCR: 常规提取RNA, 行RT-PCR, 以GAPDH为内参照, 引物序列RASSF1A: F 5'-GCATGCCTGAACTACATAAC-3, R 5'-CCACAGGCCCCACTGGCCCTG-3', 扩增产物220 bp; CylinD1: F 5'-CTGCCACCCTCTTGTCTT-3', R 5'-TCCAATGGTCCAAACTGA-3'; 扩增产物326 bp; GAPDH: F 5'-GATGCCCCCATGTTCGTCATGG-3', R 5'-ACCTTGGCCAGGGGTGCTAAG-3', 扩增产物110 bp. 扩增产物置20 g/L琼脂糖电泳, 紫外灯下观察, 凝胶分析系统拍照.
1.2.2 Western blot检测: 提取组织总蛋白, 用BCA蛋白定量试剂盒测定. 等量蛋白上样, 电泳, 转PVDF膜, 封闭, TBST洗涤, 依次与一抗(1:500)、辣根过氧化物酶(HRP)标记的羊抗鼠IgG(1:500)反应, 化学发光法(ECL)曝光显影, 冲洗胶片, GAPDH为内参照.
统计学处理 多个样本及两样本之间比较采用χ2检验, 两者之间相关性采用配对四格表资料相关性分析, 以P<0.05为差异有统计学意义.
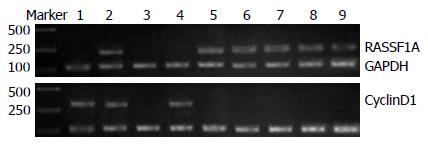
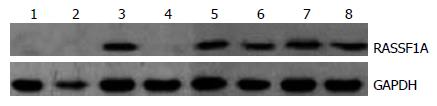
RASSF1A mRNA表达在不典型增生组低于正常组织(χ2 = 4.444, P = 0.035), 而高于腺癌组(χ2 = 9.644, P = 0.002); 尽管腺瘤组中有1例阴性表达, 但与正常组织组比较差异无显著性(P =1.000); 腺瘤组表达高于腺癌组(χ2 = 17.952, P = 0.001), 也高于不典型增生组但差别无显著性(χ2 = 2.057, P = 0.151); 正常组织组明显高于腺癌组表达(P = 0. 001). 腺癌中CyclinD1 mRNA表达高于不典型增生组(χ2 = 15.313, P = 0. 001), 也高于腺瘤组及正常组织组(P<0.01); 不典型增生组高于腺瘤组(χ2 = 25.545, P = 0.001)及正常组织组(P<0.01); 腺瘤组与正常组织组差异无统计学差异(P>0.05, 图1, 表1).
| n | RASSF1A表达 | CyclinD1表达 | |||
| n | % | n | % | ||
| 癌旁正常组织 | 20 | 20 | 100.0 | 1 | 5.0 |
| 腺瘤组 | 20 | 19 | 95.0 | 2 | 10.0 |
| 不典型增生 | 20 | 16 | 80.0 | 5 | 25.0 |
| 腺癌 | 40 | 15 | 37.5 | 31 | 77.5.0 |
RASSF1A蛋白与mRNA表达一致, 在正常组织存在RASSF1A蛋白表达, 腺瘤组中RASSF1A mRNA表达阴性的病变中蛋白表达也阴性(图2).
在胃癌组织中, RASSF1A及CyclinD1 mRNA的阳性表达与肿瘤部位、大小、TNM分期及淋巴结转移均无明显关系, 而与病理分级有关(表2).
| n | RASSF1A阳性表达 | CyclinD1阳性表达 | |||||
| n | χ2 | P | n | χ2 | P | ||
| 病变部位 | |||||||
| 贲门 | 7 | 2 | 6 | ||||
| 胃底+胃体 | 11 | 4 | 9 | ||||
| 胃窦 | 22 | 9 | 0.353 | 0.838 | 16 | 0.676 | 0.713 |
| 病变大小(cm) | |||||||
| ≥2.5 | 31 | 12 | 25 | ||||
| <2.5 cm | 9 | 3 | 0.086 | 0.769 | 6 | 0.782 | 0.377 |
| TNM分期 | |||||||
| Ⅰ-Ⅱ | 15 | 7 | 9 | ||||
| Ⅲ-Ⅳ | 25 | 8 | 0.860 | 0.354 | 21 | 2.880 | 0.090 |
| 病理分级 | |||||||
| 高分化 | 11 | 7 | 5 | ||||
| 中低分化 | 29 | 8 | 4.422 | 0.035 | 26 | 8.935 | 0.003 |
| 淋巴结转移 | |||||||
| 有 | 27 | 11 | 22 | ||||
| 无 | 13 | 4 | 0.372 | 0.542 | 9 | 0.755 | 0.385 |
RASSF1A是新近发现的抑癌基因, 其编码产物被认为是Ras的效应物, 称之为Ras相关区域家族1(ras-association domain family-1, RASSF1), Ras GTP酶是一类分子开关超家族, 通过对细胞外信号的反应来调控细胞的增殖与调亡[4,6-11]. 研究发现, RASSF1A mRNA几乎在全身所有组织中表达, 在绝大部分恶性肿瘤中表达沉默, 并认为其基因沉默在肿瘤发生中起重要作用[12-16], 本实验发现RASSF1A基因在肿瘤中阳性表达37.5%, 明显低于在正常组织及良性腺瘤中的表达, 也高于不典型增生中的表达, 且RASSF1A基因的表达与胃腺癌的分级有关, 故推测RASSF1A基因参与了肿瘤的发生发展.
CyclinD1是细胞周期G1/S期监控点重要的正向调控因子[17-21], 在G1期时, 他与CDK4/CDK6结合并使之激活, 活化的CDK4/CDK6使Rb(视网膜母细胞瘤蛋白)在G1-S转换期发生磷酸化, 释放出转录因子E2F, 进一步激活S期相关基因的转录, 推动细胞从G1期进入S期, 从而使细胞进入增殖状态[22]. CyclinD1表达异常可引起细胞周期的失控, 是细胞异常增殖和癌变的原因. 实验结果显示, 在正常组织及腺瘤组织CyclinD1表达无明显增加, 而在不典型增生组织中表达为35%, 而在腺癌中表达高达77.5%, 提示CyclinD1表达可能与胃腺癌的发生有关, 是其重要的促发因素. CyclinD1表达与CyclinD1与胃癌的部位、大小、TNM分期及淋巴结转移无关, 但与组织学分级有关, 提示其与胃腺癌的恶性分化有关, 而与胃腺癌进展的关系尚待进一步研究.
研究发现, 在胃癌组织中RASSF1A mRN和蛋白表达水平均明显降低, 62.5%的胃癌患者中RASSF1A mRNA表达缺失或明显低下. 同时发现, 胃癌组织中RASSF1A蛋白表达明显低于癌旁正常组织, 与mRNA表达水平低下一致. RASSF1A的确切抑癌机制目前尚未清楚, 有学者推测RASSF1A可能为Ras传导通路上的Ras效应物, 扮演调控细胞负生长的角色[9,23-24]. Ras传导通路在肿瘤的发生发展中扮演重要角色, 其突变在人类30%的肿瘤中都有发现, 与GTP结合的Ras蛋白家族在信号传导过程中发挥枢纽作用. 目前已证实Ras可能与下游不同的效应分子相互作用而发挥两种截然不同的功能, 促进细胞生长和分化, 又可通过诱发细胞休眠、诱导未分化和凋亡而抑制细胞生长[12,25]. 在正常细胞中, Ras的这两种效应可通过动态的平衡维系细胞正常代谢及生长, 而在肿瘤细胞中因甲基化引起的RASSF1A表达缺失可能使Ras更多地发挥促进生长效应而导致肿瘤发生[4,6,26-28]. 亦有学者发现, RASSF1A的外源性表达使肿瘤细胞中CyclinD1的累积明显受抑而减少, 从而阻断了由CyclinD1介导的细胞增殖通路, 使细胞周期阻断在G1-S期[29-31], 胃腺癌中RASSF1A mRNA与CyclinD1表达呈负相关. 提示RASSF1A可能通过拮抗CyclinD1而发挥抑癌作用, 总之, 在胃腺癌中存在着RASSF1A的表达缺失和CyclinD1的高表达, 二者之间的表达具有相关性, 且表达异常在恶性组织学发生之前即已出现, 提示在胃癌前病变及腺癌中进行两者的联合检测, 能为胃腺癌的早期筛查、诊断提供有一定的帮助, 而干预RASSF1A基因的表达有可能在胃腺癌的防治中起一定作用.
RASSF1A是新近发现的抑癌基因, CyclinD1是细胞周期重要的调控因子. 本课题旨在研究RASSF1A 及CyclinD1 mRNA在胃癌及癌前病变中的表达, 探讨RASSF1A基因与胃癌发生发展的关系.
王正康, 教授, 北京中日友好医院普外科
RASSF1A在恶性肿瘤的失活机制及抑制RASSF1A失活是否会对肿瘤的预防或治疗起一定作用是目前的研究重点.
研究发现, RASSF1A的mRNA几乎在全身所有组织中表达, 在绝大部分恶性肿瘤中表达沉默, 并认为其基因沉默在肿瘤发生中起重要作用. CyclinD1是细胞周期G1/ S期监控点重要的正向调控因子推动细胞从G1期进入S期, 从而使细胞进入增殖状态.
本研究采用RT-PCR及Western blot检测RASSF1A及CyclinD1在胃癌及癌前病变中的表达, 探讨RASSF1A及CyclinD1与胃癌发生发展的关系.
在胃癌前病变及腺癌中进行RASSF1A及CyclinD1的联合检测, 能为胃腺癌的早期筛查、诊断提供有一定的帮助.
本文提供了RASSF1A在胃癌中表达的信息, 有一定参考价值.
编辑: 李军亮 电编: 郭海丽
| 1. | Parkin DM, Bray FI, Devesa SS. Cancer burden in the year 2000. The global picture. Eur J Cancer. 2001;37 Suppl 8:S4-S66. [PubMed] [DOI] |
| 2. | Leon-Barua R, Recavarren-Arce S, Ramirez-Ramos A, Gilman RH. The Peruvian contribution to the knowledge of the role of Helicobacter pylori infection in the genesis of gastric premalignant lesions that predispose to gastric cancer. Gastroenterology. 2008;134:894. [PubMed] [DOI] |
| 3. | Massarrat S. Georg Ernst Konjetzny, German surgeon of the 20th century: a great pioneer who suggested the bacterial genesis of gastritis and its relationship to peptic ulcer and gastric cancer. Am J Gastroenterol. 2003;98:1899-1900. [PubMed] [DOI] |
| 4. | Dammann R, Yang G, Pfeifer GP. Hypermethylation of the cpG island of Ras association domain family 1A (RASSF1A), a putative tumor suppressor gene from the 3p21.3 locus, occurs in a large percentage of human breast cancers. Cancer Res. 2001;61:3105-3109. [PubMed] |
| 6. | Pijnenborg JM, Dam-de Veen GC, Kisters N, Delvoux B, van Engeland M, Herman JG, Groothuis PG. RASSF1A methylation and K-ras and B-raf mutations and recurrent endometrial cancer. Ann Oncol. 2007;18:491-497. [PubMed] [DOI] |
| 7. | Yoo YA, Na AR, Lee MS, Yoon S, Kim JS, Yoo YD. RASSF1A suppresses oncogenic H-Ras-induced c-Jun N-terminal kinase activation. Int J Oncol. 2006;29:1541-1547. [PubMed] |
| 8. | Song MS, Chang JS, Song SJ, Yang TH, Lee H, Lim DS. The centrosomal protein RAS association domain family protein 1A (RASSF1A)-binding protein 1 regulates mitotic progression by recruiting RASSF1A to spindle poles. J Biol Chem. 2005;280:3920-3927. [PubMed] [DOI] |
| 9. | Ng MH, Lau KM, Wong WS, To KW, Cheng SH, Tsang KS, Chan NP, Kho BC, Lo KW, Tong JH. Alterations of RAS signalling in Chinese multiple myeloma patients: absent BRAF and rare RAS mutations, but frequent inactivation of RASSF1A by transcriptional silencing or expression of a non-functional variant transcript. Br J Haematol. 2003;123:637-645. [PubMed] [DOI] |
| 10. | Agathanggelou A, Bieche I, Ahmed-Choudhury J, Nicke B, Dammann R, Baksh S, Gao B, Minna JD, Downward J, Maher ER. Identification of novel gene expression targets for the Ras association domain family 1 (RASSF1A) tumor suppressor gene in non-small cell lung cancer and neuroblastoma. Cancer Res. 2003;63:5344-5351. [PubMed] |
| 11. | Dammann R, Schagdarsurengin U, Liu L, Otto N, Gimm O, Dralle H, Boehm BO, Pfeifer GP, Hoang-Vu C. Frequent RASSF1A promoter hypermethylation and K-ras mutations in pancreatic carcinoma. Oncogene. 2003;22:3806-3812. [PubMed] [DOI] |
| 12. | Dammann R, Schagdarsurengin U, Seidel C, Strunnikova M, Rastetter M, Baier K, Pfeifer GP. The tumor suppressor RASSF1A in human carcinogenesis: an update. Histol Histopathol. 2005;20:645-663. [PubMed] |
| 13. | Chen YJ, Tang QB, Zou SQ. Inactivation of RASSF1A, the tumor suppressor gene at 3p21.3 in extrahepatic cholangiocarcinoma. World J Gastroenterol. 2005;11:1333-1338. [PubMed] |
| 14. | Xing XS, Huang LM, Ma XD. [Progress of RASSF1A gene in neoplasms]. Zhonghua Binglixue Zazhi. 2004;33:562-564. [PubMed] |
| 15. | Song MS, Song SJ, Ayad NG, Chang JS, Lee JH, Hong HK, Lee H, Choi N, Kim J, Kim H. The tumour suppressor RASSF1A regulates mitosis by inhibiting the APC-Cdc20 complex. Nat Cell Biol. 2004;6:129-137. [PubMed] [DOI] |
| 16. | Hesson L, Bieche I, Krex D, Criniere E, Hoang-Xuan K, Maher ER, Latif F. Frequent epigenetic inactivation of RASSF1A and BLU genes located within the critical 3p21.3 region in gliomas. Oncogene. 2004;23:2408-2419. [PubMed] [DOI] |
| 17. | Yan KX, Liu BC, Shi XL, You BR, Xu M. Role of cyclinD1 and CDK4 in the carcinogenesis induced by silica. Biomed Environ Sci. 2005;18:286-296. [PubMed] |
| 18. | Qiao LF, Xu YJ, Liu XS, Xie JG, Wang J, Du CL, Zhang J, Ni W, Chen SX. PKC promotes proliferation of airway smooth muscle cells by regulating cyclinD1 expression in asthmatic rats. Acta Pharmacol Sin. 2008;29:677-686. [PubMed] [DOI] |
| 19. | Li X, Hao Z, Fan R, Zou X, Jin H, Pan Y, He L, Du R, Gao L, Liu D. CIAPIN1 inhibits gastric cancer cell proliferation and cell cycle progression by downregulating CyclinD1 and upregulating P27. Cancer Biol Ther. 2007;6:1539-1545. [PubMed] |
| 20. | Moghaddam SJ, Haghighi EN, Samiee S, Shahid N, Keramati AR, Dadgar S, Zali MR. Immunohistochemical analysis of p53, cyclinD1, RB1, c-fos and N-ras gene expression in hepatocellular carcinoma in Iran. World J Gastroenterol. 2007;13:588-593. [PubMed] |
| 21. | Cai FG, Xiao JS, Ye QF. Effects of ischemic preconditioning on cyclinD1 expression during early ischemic reperfusion in rats. World J Gastroenterol. 2006;12:2936-2940. [PubMed] |
| 22. | Roue G, Pichereau V, Lincet H, Colomer D, Sola B. Cyclin D1 mediates resistance to apoptosis through upregulation of molecular chaperones and consequent redistribution of cell death regulators. Oncogene. 2008;27:4909-4920. [PubMed] [DOI] |
| 23. | Dammann R, Li C, Yoon JH, Chin PL, Bates S, Pfeifer GP. Epigenetic inactivation of a RAS association domain family protein from the lung tumour suppressor locus 3p21.3. Nat Genet. 2000;25:315-359. [PubMed] [DOI] |
| 24. | van Engeland M, Roemen GM, Brink M, Pachen MM, Weijenberg MP, de Bruine AP, Arends JW, van den Brandt PA, de Goeij AF, Herman JG. K-ras mutations and RASSF1A promoter methylation in colorectal cancer. Oncogene. 2002;21:3792-3795. [PubMed] [DOI] |
| 25. | Dammann R, Schagdarsurengin U, Strunnikova M, Rastetter M, Seidel C, Liu L, Tommasi S, Pfeifer GP. Epigenetic inactivation of the Ras-association domain family 1 (RASSF1A) gene and its function in human carcinogenesis. Histol Histopathol. 2003;18:665-677. [PubMed] |
| 26. | Kee SK, Lee JY, Kim MJ, Lee SM, Jung YW, Kim YJ, Park JY, Bae HI, Hong HS, Yun YK. Hypermethylation of the Ras association domain family 1A (RASSF1A) gene in gallbladder cancer. Mol Cells. 2007;24:364-371. [PubMed] |
| 27. | Liu Y, Gao W, Siegfried JM, Weissfeld JL, Luketich JD, Keohavong P. Promoter methylation of RASSF1A and DAPK and mutations of K-ras, p53, and EGFR in lung tumors from smokers and never-smokers. BMC Cancer. 2007;7:74. [PubMed] [DOI] |
| 28. | Spugnardi M, Tommasi S, Dammann R, Pfeifer GP, Hoon DS. Epigenetic inactivation of RAS association domain family protein 1 (RASSF1A) in malignant cutaneous melanoma. Cancer Res. 2003;63:1639-1643. [PubMed] |
| 29. | Whitehurst AW, Ram R, Shivakumar L, Gao B, Minna JD, White MA. The RASSF1A tumor suppressor restrains anaphase-promoting complex/cyclosome activity during the G1/S phase transition to promote cell cycle progression in human epithelial cells. Mol Cell Biol. 2008;28:3190-3197. [PubMed] [DOI] |
| 30. | Lassaletta L, Patron M, Gonzalez T, Martinez-Glez V, Rey JA, Gavilan J. RASSF1A methylation and cyclin D1 expression in vestibular schwannomas. Acta Neuropathol. 2007;114:431-433. [PubMed] [DOI] |
| 31. | Shivakumar L, Minna J, Sakamaki T, Pestell R, White MA. The RASSF1A tumor suppressor blocks cell cycle progression and inhibits cyclin D1 accumulation. Mol Cell Biol. 2002;22:4309-4318. [PubMed] [DOI] |