修回日期: 2008-04-23
接受日期: 2008-05-21
在线出版日期: 2008-06-28
胰腺导管内乳头状黏液性肿瘤(pancreatic intraepithelial neoplasias, IPMNs)是新近被认识的一种胰腺囊性肿瘤. 在不同类型胰腺肿瘤中, IPMNs预后相对较好, 具有与一般胰腺肿瘤不同的分子及临床病理特征; 按照乳头状结构及黏蛋白的表达又可将其分为多个亚型, 不同亚型又具有不同的病理特点. 病理学家提出IPMNs是胰腺癌发生过程中的重要阶段, 深入研究IPMNs及其不同亚型的病理特点及其所蕴含的分子变化, 将能更好的揭示IPMNs的发病机制及生物学特征. 本文回顾相关文献, 从分子特征、病理特征、诊断治疗及预后判断等不同角度对目前IPMNs的研究进展作一综述.
引文著录: 王伟, 李兆申, 高军. 胰腺导管内乳头状黏液性肿瘤研究进展. 世界华人消化杂志 2008; 16(18): 2025-2030
Revised: April 23, 2008
Accepted: May 21, 2008
Published online: June 28, 2008
Intraductal papillary mucinous neoplasms (IPMNs) are a well-characterized group of intraductal mucin-producing cystic neoplasms of pancreas with clear malignant potential. It has a favorable prognosis and distinct molecular or clicinopathologic features compared with other types of pancreatic tumors. In addition, IPMNs can be divided into several subtypes in accordance with the papillary structure and mucin expression profile. Importantly, as IPMNs are acknowledged as key stages during pancreatic carcinogenesis, it can better reveal the mechanism and biological characteristics of IPMNs through further investigation of the pathologic features and the molecular aberrations harbored in different subtypes of IPMNs. This paper reviewed relevant literatures and elucidated its progression in terms of the molecular and clicinopathologic characteristics, diagnosis, treatment and prognosis of IPMNs.
- Citation: Wang W, Li ZS, Gao J. Advances in pancreatitic intraductal papillary mucinous neoplasms. Shijie Huaren Xiaohua Zazhi 2008; 16(18): 2025-2030
- URL: https://www.wjgnet.com/1009-3079/full/v16/i18/2025.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v16.i18.2025
近年来, 病理学家提出胰腺上皮内瘤变(pancreatic intraepithelial neoplasias, PanINs)和胰腺导管内乳头状黏液性肿瘤(intraductal papillary mucinous neoplasms, IPMNs)是胰腺癌发生过程中的重要阶段, 为胰腺癌早期研究提供了新靶点[1-5]. PanINs和IPMNs在组织病理学上呈现出逐渐增加的细胞及组织结构异型表现. 而在分子病理变化上, PanINs、IPMNs和胰腺导管腺癌(pancreatic ductal adenocarcinoma, PDAC)之间存在相似性[6-8]. 越来越多的研究结果表明: 在由正常胰腺导管→PanIN-1A→PanIN-1B→PanIN-2→PanIN-3→PDAC以及正常胰腺导管→IPMA→IPMB→IPMC的逐级进展过程中, 多基因共同参与导致上皮细胞的细胞保护机制破坏等以致肿瘤的发生[9-13]. 这些研究证实了高级别PanINs和IPMNs是胰腺癌的癌前病变, 对其分子特征的深入研究必然进一步揭示胰腺癌的发病机制. 本文回顾了相关文献, 重点阐述了IPMNs及其不同亚型的分子及临床病理特征、诊断治疗及预后判断, 以供参考.
胰腺导管内乳头状黏液性肿瘤是最近几年被认识的一种胰腺囊性肿瘤. 早在1982年, Ohhashi et al[14]便提出胰腺导管内乳头状黏液性肿瘤: 一种发生于胰腺导管内的独特病变. 以后曾陆续有一些报道对该肿瘤进行不同的命名, 如: 产黏液癌、高分泌黏液癌、导管内乳头状肿瘤、导管高分泌黏液肿瘤、导管内癌、导管产黏液肿瘤、导管扩展型黏液性囊腺瘤和囊腺癌、黏液性导管扩展症、胰管扩展型产黏液肿瘤、胰腺导管内乳头状黏液性肿瘤等[15-19]. 1996年, WHO给出了IPMNs的分级诊断标准以区别于其他一些胰腺产黏液肿瘤如胰腺黏液性囊性腺瘤或囊腺癌[20]. 2003美国Johns Hopkins医院制定了目前国际一致认同的PanINs和IPMNs分级诊断标准[1]. 2006年国际胰腺病学协会(international association of pancreatology, IAP)又出台了新的标准以鉴别IPMNs和胰腺黏液性囊性瘤(mucinous cystic neoplasms, MCNs)[21].
IPMNs指发生于主胰管或分支胰管内由上皮细胞瘤性生长形成大体可见的、乳头状(偶见扁平状)、产黏蛋白的肿瘤, 并伴随有不同程度的胰管扩张, 常见病变直径>1 cm, 病变中包含多种类型细胞, 并伴有不同程度的细胞及组织结构异型性.
可分为IPMN腺瘤(IPMN adenoma, IPMNa): 形成规则的乳头结构, 细胞核无明显异型, 核分裂几乎不存在; IPMN交界瘤(IPMN borderline, IPMNb): 不规则的乳头结构, 细胞中度异型, 胞核形状不一, 核分裂多见; IPMN黏液癌(IPMN carcinoma, IPMNc): 重度细胞异型, 上皮出现不规则的芽突和间桥表现, 同时根据是否累及导管周围胰腺组织, 可将IPMNc分为浸润癌和非浸润癌[1].
主胰管型(main duct type), 主胰管扩张而肿瘤主要存在于主胰管内; 分支胰管型(branch duct type), 分支胰管扩张而肿瘤不存在于主胰管; 混合型(combined type), 肿瘤既存在于主胰管又存在于分支胰管. 主胰管型IPMNs增殖活性大, 表现为大的病灶, 镜下常表现为肠型上皮特征, 分级多为交界瘤或原位癌, 进展为浸润癌的机率较大(25%-50%); 分支胰管型IPMNs表现为小病灶、导管轻度囊性扩张, 镜下表现为胃型上皮特征, 分级常为腺瘤, 进展为癌的机率较小(<20%)[22]. 主胰管型IPMNs胰头分布(57%)多于胰体尾(43%), 分支胰管型IPMNs以胰头分布为主(93%)[23].
2.3.1 分型进展: 1999年Yonezawa et al[24]提出IPMNs可分为两型: 乳头状透明细胞型和绒毛状暗细胞型. 2002年Yonezawa et al[25-26]又提出IPMNs可分为三型: 透明细胞型, 暗细胞以及密集细胞型, 同年, Adsay et al[27-28]提出IPMNs可分为两型: 肠型和胰胆管型. 2004年Adsay et al[29]提出IPMNs可分为四型: 胃型(裸型)、肠型、胰胆管型和未分类型. 2005年Furukawa et al[30]提出IPMN可分为: 胃型、肠型、胰胆管型和嗜酸细胞型. 不同分型之间存在一定的对应性, 即透明细胞型一般为胃型, 暗细胞型为肠型, 而密集细胞型对应嗜酸细胞型. 其中, 胃型和肠型是公认的两个主要类型, 而胰胆管型和嗜酸细胞型较少见.
2.3.2 不同亚型的形态特征: 胃型: 瘤性上皮由透明或轻度嗜酸的立方或柱状细胞形成, 胞核呈圆形或卵圆形, 细胞排列少有假复层结构出现, 类似于胃小凹上皮. 表现为轻度异型. 肠型: 瘤性上皮由暗嗜酸细胞或透明柱状细胞形成, 胞核呈卵圆形或梭形, 细胞排列常出现假复层结构. 形成类似于肠绒毛状腺瘤的绒毛状或乳头状结构. 常表现为中度到重度异型. 胰胆管型: 复杂的乳头状结构被覆核大、偏心的立方细胞, 类似于胆道乳头状肿瘤, 表现为重度异型. 嗜酸细胞型: 又称为"导管内嗜酸性乳头状瘤"(intraductal oncocytic papillary neoplasm, IOPN), 罕见, 常为重度异型[29].
2.3.3 不同亚型的病理特征: 胃型常表现为轻度异型, 病理分级主要为腺瘤, 其进展为浸润癌的发生率较低, 常形成较小的肿瘤, 肿瘤成分中常出现幽门腺样结构和低级别瘤变样复合体结构; 肠型表现为中度到重度异型, 病理分级主要为交界瘤和原位癌, 其进展为浸润癌的发生率较高, 常形成较大的肿瘤, 而幽门腺样结构和低级别瘤变样复合体结构少见; 胰胆管型常表现为重度异型, 病理分级主要为原位癌, 其进展为浸润癌的发生率较高[29,31]. 不同亚型进展形成的肿瘤类型也不相同, 肠型常进展为浸润性胶样癌(invasive colloid carcinomas), 而胰胆管型为浸润性管型癌(invasive tubular carcinomas)(表1).
| HE分型 | 病理分级(%) | 浸润癌发生率(%) | 浸润癌类型 | 肿瘤直径(cm) | 幽门腺样结构(%) | 低级别PanIN复合体(%) | 间质萎缩 | ||
| 腺瘤 | 交界瘤 | 原位癌 | |||||||
| 胃型 | 48 | 26 | 26 | 17 | - | 2.6 | 96 | 82 | 少见 |
| 肠型 | 0 | 15 | 85 | 62 | 浸润性胶样癌 | 5.5 | 33 | 3 | 常见 |
| 胰胆管 | 0 | 6 | 94 | 56 | 浸润性管型癌 | 4.0 | - | - | - |
2.3.4 不同亚型的分子差异: 许多针对IPMNs黏蛋白(Mucins)表达的研究证明IPMNs主要存在三种组织类型, 主要存在三种组织类型, 即胃型、肠型和胰胆管型, 他们在MUC1、MUC2和MUC5AC的表达上存在一定的排他性表型, 这些不同组织类型的IPMNs往往进展为病理学上不同的胰腺癌类型[32-34]. 尾侧型同源转录因子2(CDX2)是人体肠道中特异表达的核转录因子, CDX2在食管和正常胃黏膜上皮中不表达[35]. 在IPMNs亚型中CDX2主要表达于肠型[29], 再次证实IPMNs不同亚型具有不同的组织起源(表2).
| 组织分型 | MUC1 | MUC2 | MUC5AC | CDX2 |
| 正常胰腺 | + | - | + | + |
| 导管上皮 | ||||
| 胃型 | - | - | + | - |
| 肠型 | - | + | ± | + |
| 胰胆管型 | + | - | ± | - |
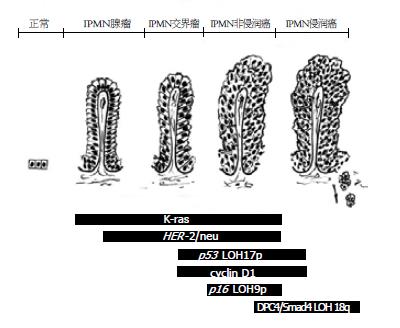
在分子病理变化上, IPMNs和PanINs、胰腺导管腺癌(pancreatic ductal adenocarcinoma, PDAC)之间存在一定的相似性. 如K-ras突变激活, HER-2/neu、P53、细胞周期素蛋白cyclin D1高表达, p16NK4A缺失表达等[6]. K-ras突变激活是IPMNs进展过程中的早期事件, 其发生率随组织异型程度增高而增加[8,36]. 而CEA、CA-199和DUPAN-2的表达在IPMNs和浸润癌中基本相当+. 免疫组化法检测增殖活性指标Ki67和PCNA在正常胰腺导管、IPMNs及浸润癌中的表达, 结果表明随着组织异型程度增高其表达渐进性增强[38]. 同样, 细胞周期调节基因突变率在IPMNs中随异型程度增高而增加, 并接近于相同异型程度的PanINs(PanIN-1 vs IPMNa, PanIN-2 vs IPMNb, PanIN-3 vs IPMNc)[39]. 这些研究证明了伴随IPMNs组织学逐级进展所发生的微观分子变化(图1), 从分子水平证实IPMNs是胰腺癌的癌前病变[40].
不同的是, IPMNs起源的胰腺癌很少有DPC4/Smad4基因缺失表达, 而DPC4/Smad4基因在非IPMNs起源的胰腺癌中其缺失表达率为55%, 在PanINs中为30%[40-42]. 其次, 约1/3的IPMNs存在Peutz-Jegher综合征基因STK11/LKB1失活, 而PanINs及PDAC中少见[43]. 另外, 黏蛋白家族在胰腺组织中的表达也可以证明IPMNs和PanINs是两种不同类型的病变, 并具有不同的恶性进展途径. PanINs→PDCA进展途径, 主要表现为MUC1阳性表达而MUC2阴性表达, MUC1和MUC2在不同级别PanINs中的阳性表达率为: PanIN-1, 2 MUC1(10%), MUC2(0%); PanIN-3 MUC1(61%), MUC2(0%); PDAC MUC1(63%), MUC2(1%). IPMNs→胶质癌进展途径: 主要表现为MUC1阴性表达而MUC2阳性表达, MUC1和MUC2在不同级别IPMNs中的阳性表达率为: IPMA, B MUC1(8%), MUC2(31%); IPMC MUC1(20%), MUC2(54%); 侵袭性胶质癌 MUC1(0%), MUC2(100%)[27].
随着影像学检查水平的提高, 越来越多的无症状性IPMNs被早期发现. 这种无症状的IPMNs常为良性病变, 在其进展为侵袭性肿瘤之前可被有效治疗. 并且大量研究证明非侵袭性IPMNs患者的预后显著优于侵袭性IPMNs患者, 因此准确判断IPMNs的良恶性、严密随访IPMNs的进展将有效指导临床治疗及预后判断. Kawamoto et al分析了46位IPMNs患者的胰腺CT资料, 发现主胰管受累、主胰管扩张、弥漫性或多发性病灶、壁内结节、肿瘤大小、胰管阻塞等都可作为判断肿瘤恶性行为的指标[44]. Sahani et al提出肿瘤大于5 cm, 主胰管直径大于15 mm, 管壁结节大于10 mm提示恶性[45]. Kubo et al研究认为, 主胰管型IPMNs, 扩张胰管直径大于10 mm, 分支型, 肿瘤直径大于40 mm, 肿瘤壁结节大于10 mm, 高度提示恶性[46]. 另外, 黏蛋白在IPMNs中的表达除了对IPMNs分型有重要意义, 而且对IPMNs的鉴别诊断及生物学行为判断具有重要作用. MUC1阳性提示侵袭性表型, MUC2阳性提示惰性表型. MUC1过表达是提示侵袭性IPMNs最敏感、最特异的标记[27,47]. MUC2对诊断IPMNs虽然特异, 但敏感性不强. 常规肿瘤标志物CEA、CA199在这种早期IPMNs中常为正常水平[48]. 新近研究发现, 染色体倍性形态与病变的生物学行为, 恶性倾向及预后有关, 染色体成二倍型提示良性病变[6].
IPMNs一旦诊断明确, 手术切除仍是首选的治疗方法, 但并非所有的IPMNs患者均需手术治疗, 不同IPMNs亚型的治疗方案不同. Tanaka et al[4]建议所有的主胰管型和混合型IPMNs应手术治疗. 而分支型IPMNs的治疗相对复杂, 通常认为, 小于1 cm的病变可保守治疗并定期MRI或CT随访; 1-3 cm的病变应进行EUS联合MRCP或ERCP检查综合判断; 主胰管直径增加大于6 mm并有壁内结节的高风险患者应手术切除. 而非手术治疗患者的随访周期取决于病灶大小, 如果病灶小于10 mm则随访周期为1年; 10-20 mm的病灶, 随访周期为6-12 mo; 大于20 mm的病灶, 随访周期为3-6 mo.
IPMNs手术治疗方法包括全胰切除术、保留幽门的胰十二指肠切除术、保留十二指肠胰头切除术、保留脾脏的胰体尾切除及胰腺部分切除术等. 最佳手术方式的选择取决于对病变部位及病变程度的准确判断. 但目前很难正确判断肿瘤在胰管内的侵犯范围, 主要依靠术中切除标本的快速冰冻结果. 此方法适用于主胰管型和混合型, 因为大部分肿瘤在胰管内呈连续生长. 相反, 分支型经常为多灶性和低度恶性, 因此应进行局部切除.
判断IPMNs预后的因素主要包括: 组织学类型、浸润成分、淋巴结转移、血管浸润、神经浸润、肿瘤大小、是否有壁内结节及胰腺导管扩张程度. 总的来讲, IPMNs预后较好, IPMNs进展为胰腺癌的总的发生率是10%-20%; IPMNs术后5年生存率>55%[27], 明显高于PDAC, 其中非浸润癌术后5年生存率为88%, 而浸润癌为36%[23]. 一般认为, 主胰管型和混和型较分支型IPMNs恶性程度高, 但有研究认为主胰管型和分支胰管型的预后无统计学差异[49]. 另有研究认为, 虽然IPMNs常常为单一病灶, 但仍有30%的IPMNs表现为多病灶, 这就意味着单一病灶可发展为多病灶, 并且良性IPMNs也会进展为恶性, 定期影像检查可监测良性IPMNs进展至恶性肿瘤的过程, 如经腹超声检查发现主胰管直径增加大于2.2 mm/年, 或囊腔直径增加大于11.3 mm/年, 或壁内结节增大超过3.3 mm/年以及新出现的壁内结节时, 可高度怀疑恶性肿瘤并及时进行有效治疗[50].
在不同类型胰腺肿瘤中, IPMNs预后相对较好, 具有与一般胰腺肿瘤不同的分子及临床病理特征; 按照乳头状结构及黏蛋白的表达特征又可将其分为多个亚型, 每种亚型又具有各自的临床病理特征, 这些证据充分说明了胰腺肿瘤组织起源及分子病理机制的多样性, 提示我们在针对胰腺肿瘤的研究特别是IPMNs的研究中要深入探讨IPMNs不同亚型的特点及其所蕴含的分子病理变化, 将能更好的揭示IPMNs的发病机制及生物学特征. 关于IPMNs的诊疗特别是对早期病变的诊断时于我们要建立有足够敏感性和特异性的方法在无症状患者中进行筛查, 以尽可能的发现早期病变, 并采取有效治疗, 改善预后.
在不同类型胰腺肿瘤中, IPMNs预后相对较好, 具有与一般胰腺肿瘤不同的分子及临床病理特征, 病理学家提出IPMNs是胰腺癌发生过程中的重要阶段, 深入研究IPMNs及其不同亚型的病理特点及其所蕴含的分子变化, 将能更好的揭示IPMNs的发病机制及生物学特征. 本文回顾了相关文献, 从分子特征、病理特征、诊断治疗及预后判断等不同角度对目前IPMNs的研究进展作一综述.
张学, 教授, 中国协和医科大学基础医学院医学遗传学系.
IPMNs按乳头形态特征可分为不同亚型, 不同亚型黏蛋白的表达谱证实不同亚型具有不同的组织起源, 深入研究不同亚型之间的分子差异将有助于揭示IPMNs的发病机制.
本文详细阐述了IPMNs及其不同亚型的分子及临床病理特征, 同时又回顾了当前IPMNs临床诊断及预后判断的研究进展.
文中IPMNs的最新分型及其分子特征对胰腺肿瘤研究有重要指导意义; IPMNs的诊断、良恶性鉴别及预后判断方法给临床诊疗工作提供了有力工具.
染色体倍性: 细胞中包含的染色体组数或基因组数. 正常的配子细胞中所包含的染色体数或半数的体细胞染色体数称为一套单倍染色体,用符号n表示. 某种生物的完整的一套单倍染色体称为该种生物的基因组或染色体组. 具有一个染色体组的细胞和由这样的细胞组成的个体称为单倍体(n), 具有两个染色体组的细胞或个体称为二倍体(2n), 具有两个以上整套染色体组的细胞或个体则称为多倍体, 包括三倍体(3n)、四倍体(4n)等. 由相同来源染色体组形成的多倍体称为同源多倍体, 由不同来源不同染色体组形成的多倍体称为异源多倍体. 此外, 具有不成套染色体组的细胞或个体称为非整倍体或异倍体(见染色体畸变).
本文信息量较大, 图文并茂, 具有可读性, 但学术价值一般.
编辑: 李军亮 电编:何基才
| 1. | Takaori K, Hruban RH, Maitra A, Tanigawa N. Pancreatic intraepithelial neoplasia. Pancreas. 2004;28:257-262. [PubMed] [DOI] |
| 3. | Maitra A, Fukushima N, Takaori K, Hruban RH. Precursors to invasive pancreatic cancer. Adv Anat Pathol. 2005;12:81-91. [PubMed] [DOI] |
| 4. | Tanaka M, Chari S, Adsay V, Fernandez-del Castillo C, Falconi M, Shimizu M, Yamaguchi K, Yamao K, Matsuno S. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology. 2006;6:17-32. [PubMed] [DOI] |
| 5. | Takaori K. Current understanding of precursors to pancreatic cancer. J Hepatobiliary Pancreat Surg. 2007;14:217-223. [PubMed] [DOI] |
| 6. | Biankin AV, Kench JG, Dijkman FP, Biankin SA, Henshall SM. Molecular pathogenesis of precursor lesions of pancreatic ductal adenocarcinoma. Pathology. 2003;35:14-24. [PubMed] [DOI] |
| 7. | Luttges J, Galehdari H, Brocker V, Schwarte-Waldhoff I, Henne-Bruns D, Kloppel G, Schmiegel W, Hahn SA. Allelic loss is often the first hit in the biallelic inactivation of the p53 and DPC4 genes during pancreatic carcinogenesis. Am J Pathol. 2001;158:1677-1683. [PubMed] |
| 8. | Yoshizawa K, Nagai H, Sakurai S, Hironaka M, Morinaga S, Saitoh K, Fukayama M. Clonality and K-ras mutation analyses of epithelia in intraductal papillary mucinous tumor and mucinous cystic tumor of the pancreas. Virchows Arch. 2002;441:437-443. [PubMed] [DOI] |
| 9. | Albazaz R, Verbeke CS, Rahman SH, McMahon MJ. Cyclooxygenase-2 expression associated with severity of PanIN lesions: a possible link between chronic pancreatitis and pancreatic cancer. Pancreatology. 2005;5:361-369. [PubMed] [DOI] |
| 10. | Klein WM, Hruban RH, Klein-Szanto AJ, Wilentz RE. Direct correlation between proliferative activity and dysplasia in pancreatic intraepithelial neoplasia (PanIN): additional evidence for a recently proposed model of progression. Mod Pathol. 2002;15:441-447. [PubMed] [DOI] |
| 11. | Argani P, Iacobuzio-Donahue C, Ryu B, Rosty C, Goggins M, Wilentz RE, Murugesan SR, Leach SD, Jaffee E, Yeo CJ. Mesothelin is overexpressed in the vast majority of ductal adenocarcinomas of the pancreas: identification of a new pancreatic cancer marker by serial analysis of gene expression (SAGE). Clin Cancer Res. 2001;7:3862-3868. [PubMed] |
| 12. | Swartz MJ, Batra SK, Varshney GC, Hollingsworth MA, Yeo CJ, Cameron JL, Wilentz RE, Hruban RH, Argani P. MUC4 expression increases progressively in pancreatic intraepithelial neoplasia. Am J Clin Pathol. 2002;117:791-796. [PubMed] [DOI] |
| 13. | Rosty C, Ueki T, Argani P, Jansen M, Yeo CJ, Cameron JL, Hruban RH, Goggins M. Overexpression of S100A4 in pancreatic ductal adenocarcinomas is associated with poor differentiation and DNA hypomethylation. Am J Pathol. 2002;160:45-50. [PubMed] |
| 14. | Ohhashi K, Murakami Y, Takekoshi T. Four cases of mucin producing cancer of pancreas on specific findings of the papilla of Vater. Prog Dig Endosc. 1982;20:348-351. |
| 15. | Wiesenauer CA, Schmidt CM, Cummings OW, Yiannoutsos CT, Howard TJ, Wiebke EA, Goulet RJ Jr, McHenry L, Sherman S, Lehman GA, Cramer H, Madura JA. Preoperative predictors of malignancy in pancreatic intraductal papillary mucinous neoplasms. Arch Surg. 2003;138:610-617; discussion 617-618. [PubMed] [DOI] |
| 16. | Chari ST, Yadav D, Smyrk TC, DiMagno EP, Miller LJ, Raimondo M, Clain JE, Norton IA, Pearson RK, Petersen BT. Study of recurrence after surgical resection of intraductal papillary mucinous neoplasm of the pancreas. Gastroenterology. 2002;123:1500-1507. [PubMed] [DOI] |
| 17. | Bernard P, Scoazec JY, Joubert M, Kahn X, Le Borgne J, Berger F, Partensky C. Intraductal papillary-mucinous tumors of the pancreas: predictive criteria of malignancy according to pathological examination of 53 cases. Arch Surg. 2002;137:1274-1278. [PubMed] [DOI] |
| 18. | Maire F, Hammel P, Terris B, Paye F, Scoazec JY, Cellier C, Barthet M, O'Toole D, Rufat P, Partensky C. Prognosis of malignant intraductal papillary mucinous tumours of the pancreas after surgical resection. Comparison with pancreatic ductal adenocarcinoma. Gut. 2002;51:717-722. [PubMed] [DOI] |
| 19. | Matsumoto T, Aramaki M, Yada K, Hirano S, Himeno Y, Shibata K, Kawano K, Kitano S. Optimal management of the branch duct type intraductal papillary mucinous neoplasms of the pancreas. J Clin Gastroenterol. 2003;36:261-265. [PubMed] [DOI] |
| 20. | Klöppel G, Solcia E, Longneker DS, Capella C, Sobin LH. Histological typing of tumours of the exocrine pancreas. World Health Organization International Classification of Tumors, 2nd ed. Berlin: Springer 1996; 11-20. |
| 21. | Tanaka M, Chari S, Adsay V, Fernandez-del Castillo C, Falconi M, Shimizu M, Yamaguchi K, Yamao K, Matsuno S. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology. 2006;6:17-32. [PubMed] [DOI] |
| 22. | Adsay NV. Intraductal papillary mucinous neoplasms of the pancreas from pathogenesis to pathology. Digestive disease Week. 2005;May 16. |
| 23. | Fukushima N, Mukai K, Kanai Y, Hasebe T, Shimada K, Ozaki H, Kinoshita T, Kosuge T. Intraductal papillary tumors and mucinous cystic tumors of the pancreas: clinicopathologic study of 38 cases. Hum Pathol. 1997;28:1010-1017. [PubMed] [DOI] |
| 24. | Yonezawa S, Horinouchi M, Osako M, Kubo M, Takao S, Arimura Y, Nagata K, Tanaka S, Sakoda K, Aikou T. Gene expression of gastric type mucin (MUC5AC) in pancreatic tumors: its relationship with the biological behavior of the tumor. Pathol Int. 1999;49:45-54. [PubMed] [DOI] |
| 25. | Yonezawa S, Nakamura A, Horinouchi M, Sato E. The expression of several types of mucin is related to the biological behavior of pancreatic neoplasms. J Hepatobiliary Pancreat Surg. 2002;9:328-341. [PubMed] [DOI] |
| 26. | Nakamura A, Horinouchi M, Goto M, Nagata K, Sakoda K, Takao S, Imai K, Kim YS, Sato E, Yonezawa S. New classification of pancreatic intraductal papillary-mucinous tumour by mucin expression: its relationship with potential for malignancy. J Pathol. 2002;197:201-210. [PubMed] [DOI] |
| 27. | Adsay NV, Merati K, Andea A, Sarkar F, Hruban RH, Wilentz RE, Goggins M, Iocobuzio-Donahue C, Longnecker DS, Klimstra DS. The dichotomy in the preinvasive neoplasia to invasive carcinoma sequence in the pancreas: differential expression of MUC1 and MUC2 supports the existence of two separate pathways of carcinogenesis. Mod Pathol. 2002;15:1087-1095. [PubMed] [DOI] |
| 28. | Adsay NV. Intraductal papillary mucinous neoplasms of the pancreas: pathology and molecular genetics. J Gastrointest Surg. 2002;6:656-659. [PubMed] [DOI] |
| 29. | Adsay NV, Merati K, Basturk O, Iacobuzio-Donahue C, Levi E, Cheng JD, Sarkar FH, Hruban RH, Klimstra DS. Pathologically and biologically distinct types of epithelium in intraductal papillary mucinous neoplasms: delineation of an "intestinal]pathway of carcinogenesis in the pancreas. Am J Surg Pathol. 2004;28:839-848. [PubMed] [DOI] |
| 30. | Furukawa T, Kloppel G, Volkan Adsay N, Albores-Saavedra J, Fukushima N, Horii A, Hruban RH, Kato Y, Klimstra DS, Longnecker DS. Classification of types of intraductal papillary-mucinous neoplasm of the pancreas: a consensus study. Virchows Arch. 2005;447:794-799. [PubMed] [DOI] |
| 31. | Ban S, Naitoh Y, Mino-Kenudson M, Sakurai T, Kuroda M, Koyama I, Lauwers GY, Shimizu M. Intraductal papillary mucinous neoplasm (IPMN) of the pancreas: its histopathologic difference between 2 major types. Am J Surg Pathol. 2006;30:1561-1569. [PubMed] [DOI] |
| 32. | Moriya T, Kimura W, Semba S, Sakurai F, Hirai I, Ma J, Fuse A, Maeda K, Yamakawa M. Biological similarities and differences between pancreatic intraepithelial neoplasias and intraductal papillary mucinous neoplasms. Int J Gastrointest Cancer. 2005;35:111-119. [PubMed] [DOI] |
| 33. | Levi E, Klimstra DS, Andea A, Basturk O, Adsay NV. MUC1 and MUC2 in pancreatic neoplasia. J Clin Pathol. 2004;57:456-462. [PubMed] [DOI] |
| 34. | Yamasaki H, Ikeda S, Okajima M, Miura Y, Asahara T, Kohno N, Shimamoto F. Expression and localization of MUC1, MUC2, MUC5AC and small intestinal mucin antigen in pancreatic tumors. Int J Oncol. 2004;24:107-113. [PubMed] |
| 35. | Werling RW, Yaziji H, Bacchi CE, Gown AM. CDX2, a highly sensitive and specific marker of adenocarcinomas of intestinal origin: an immunohistochemical survey of 476 primary and metastatic carcinomas. Am J Surg Pathol. 2003;27:303-310. [PubMed] [DOI] |
| 36. | Kondo H, Sugano K, Fukayama N, Hosokawa K, Ohkura H, Ohtsu A, Mukai K, Yoshida S. Detection of K-ras gene mutations at codon 12 in the pancreatic juice of patients with intraductal papillary mucinous tumors of the pancreas. Cancer. 1997;79:900-905. [PubMed] [DOI] |
| 37. | Terada T, Ohta T, Nakanuma Y. Expression of oncogene products, anti-oncogene products and oncofetal antigens in intraductal papillary-mucinous neoplasm of the pancreas. Histopathology. 1996;29:355-361. [PubMed] [DOI] |
| 38. | Kench JG, Eckstein RP, Smith RC. Intraductal papillary-mucinous neoplasm of the pancreas: a report of five cases with immunohistochemical findings. Pathology. 1997;29:7-11. [PubMed] [DOI] |
| 39. | Fujii H, Inagaki M, Kasai S, Miyokawa N, Tokusashi Y, Gabrielson E, Hruban RH. Genetic progression and heterogeneity in intraductal papillary-mucinous neoplasms of the pancreas. Am J Pathol. 1997;151:1447-1454. [PubMed] |
| 40. | Biankin AV, Kench JG, Morey AL, Lee CS, Biankin SA, Head DR, Hugh TB, Henshall SM, Sutherland RL. Overexpression of p21(WAF1/CIP1) is an early event in the development of pancreatic intraepithelial neoplasia. Cancer Res. 2001;61:8830-8837. [PubMed] |
| 41. | Iacobuzio-Donahue CA, Klimstra DS, Adsay NV, Wilentz RE, Argani P, Sohn TA, Yeo CJ, Cameron JL, Kern SE, Hruban RH. Dpc-4 protein is expressed in virtually all human intraductal papillary mucinous neoplasms of the pancreas: comparison with conventional ductal adenocarcinomas. Am J Pathol. 2000;157:755-761. [PubMed] |
| 42. | Hruban RH, Offerhaus GJ, Kern SE, Goggins M, Wilentz RE, Yeo CJ. Tumor-suppressor genes in pancreatic cancer. J Hepatobiliary Pancreat Surg. 1998;5:383-391. [PubMed] [DOI] |
| 43. | Sahin F, Maitra A, Argani P, Sato N, Maehara N, Montgomery E, Goggins M, Hruban RH, Su GH. Loss of Stk11/Lkb1 expression in pancreatic and biliary neoplasms. Mod Pathol. 2003;16:686-691. [PubMed] [DOI] |
| 44. | Kawamoto S, Horton KM, Lawler LP, Hruban RH, Fishman EK. Intraductal papillary mucinous neoplasm of the pancreas: can benign lesions be differentiated from malignant lesions with multidetector CT? Radiographics. 2005;25:1451-1468; discussion 1468-1470. [PubMed] [DOI] |
| 45. | Sahani D, Prasad S, Saini S, Mueller P. Cystic pancreatic neoplasms evaluation by CT and magnetic resonance cholangiopancreatography. Gastrointest Endosc Clin N Am. 2002;12:657-672. [PubMed] [DOI] |
| 46. | Kubo H, Chijiiwa Y, Akahoshi K, Hamada S, Harada N, Sumii T, Takashima M, Nawata H. Intraductal papillary-mucinous tumors of the pancreas: differential diagnosis between benign and malignant tumors by endoscopic ultrasonography. Am J Gastroenterol. 2001;96:1429-1434. [PubMed] [DOI] |
| 49. | Jang JY, Kim SW, Ahn YJ, Yoon YS, Choi MG, Lee KU, Han JK, Kim WH, Lee YJ, Kim SC. Multicenter analysis of clinicopathologic features of intraductal papillary mucinous tumor of the pancreas: is it possible to predict the malignancy before surgery? Ann Surg Oncol. 2005;12:124-132. [PubMed] [DOI] |
| 50. | Yamaguchi T, Baba T, Ishihara T, Kobayashi A, Nakamura K, Tadenuma H, Ito H, Miyazaki M, Saisho H. Long-term follow-up of intraductal papillary mucinous neoplasm of the pancreas with ultrasonography. Clin Gastroenterol Hepatol. 2005;3:1136-1143. [PubMed] [DOI] |