修回日期: 2008-03-30
接受日期: 2008-04-26
在线出版日期: 2008-06-08
目的: 探讨去甲基化药物5-杂氮-2'-脱氧胞苷(5-Aza-CdR)在裸鼠体内诱导HepG2细胞T-cadherin的表达, 及其对HepG2种植瘤生长的抑制作用.
方法: 皮下接种法建立HepG2细胞裸鼠种植瘤模型, 随机分为实验组(11只)和对照组(10只). 实验组裸鼠给予5-Aza-CdR注射, 对照组裸鼠注射等量PBS. 4 wk后处死裸鼠, 观察种植瘤的生长情况, 用RT-PCR技术及免疫组织化学技术检测T-cadherin mRNA及蛋白的表达.
结果: 用药4 wk后, 实验组肿瘤组织的T-cadherin mRNA的表达水平显著高于对照组(t = 6.613, P<0.05); 对照组HepG2细胞几乎检测不到T-cadherin蛋白表达, 而实验组裸鼠种植瘤细胞膜上可检测到T-cadherin蛋白表达; 实验组肿瘤的平均体积明显小于对照组肿瘤平均体积(t = 2.337, P = 0.025).
结论: 5-Aza-CdR能诱导裸鼠皮下种植HepG2瘤细胞的T-cadherin表达, 抑制HepG2种植瘤的生长, 其机制可能为5-Aza-CdR使甲基化的T-cadherin启动子去甲基化, 恢复T-cadherin的表达, 从而抑制HepG2种植瘤生长.
引文著录: 梁治坤, 黄志勇, 陈孝平, 刘聪, 吴在德. 5-杂氮-2'-脱氧胞苷诱导裸鼠HepG2种植瘤细胞T-cadherin的表达及其对种植瘤的抑制作用. 世界华人消化杂志 2008; 16(16): 1741-1745
Revised: March 30, 2008
Accepted: April 26, 2008
Published online: June 8, 2008
AIM: To observe 5-Aza-2'-deoxycytidine (5-Aza-CdR)-induced T-cadherin gene expression in HepG2 cells and to determine its inhibitory effects on proliferation of HepG2-derived tumor cells in nude mice.
METHODS: The HepG2-derived tumor model in nude mice was established by subcutaneous inoculation. Twenty-one Nude mice were randomly divided into two groups: the experiment group (n = 11) and control group (n = 10). Experiment group nude mice were intraperitoneally injected with 5-Aza-CdR while control group nude mice were only given equivalent volume of PBS. The mice were killed at week 4. Tumor growth in nude mice was observed, and the T-cadherin mRNA and protein expressions of the tumors were detected using reverse transcription-polymerase chain reaction (RT-PCR) and immunohistochemistry.
RESULTS: At the end of the fourth week, the T-cadherin mRNA expression in the tumors of the experiment group was significantly higher than that the control group (t = 6.613, P < 0.05). Immunohistochemistry further showed that the protein expression of T-cadherin was almost not detected in the HepG2-derived tumor cells of the control group w while the T-cadherin expression was detected on the membranes of the HepG2-derived tumor cells of the experiment group. After 4 weeks' treatment with 5-Aza-CdR, the average volume of HepG2-derived tumors in the experiment group was significantly smaller than that in the control group (t = 2.337, P = 0.025).
CONCLUSION: 5-Aza-CdR induces HepG2 cells to re-express T-cadherin and inhibits growth of HepG2-derived tumors in nude mice. Its mechanism may be that demethylation of the methylated T-cadherin promoter induced by 5-Aza-CdR restores T-cadherin reexpression and thus inhibits the growth of the HepG2-derived tumors in nude mice.
- Citation: Liang ZK, Huang ZY, Chen XP, Liu C, Wu ZD. 5-Aza-2'-deoxycytidine-induced T-cadherin expression in HepG2-derived tumors and its inhibitory effects on tumor growth in nude mice. Shijie Huaren Xiaohua Zazhi 2008; 16(16): 1741-1745
- URL: https://www.wjgnet.com/1009-3079/full/v16/i16/1741.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v16.i16.1741
肝癌的发生是一个多步骤、多阶段的复杂过程, 与癌基因激活和抑癌基因失活密切相关. 近年来, 对抑癌基因失活与肿瘤发生之间关系的研究越来越受到人们的重视, 尤其是对抑癌基因启动子区CpG岛甲基化导致基因失活的研究已渐成为目前研究的热点. 以往研究发现, 对一些启动子区CpG岛甲基化而失活的抑癌基因, 应用去甲基化试剂5-杂氮-2'-脱氧胞苷(5-Aza-CdR)可使这些抑癌基因去甲基化而重新表达[1-4]. 研究已表明, 胶质母细胞瘤C6细胞缺失T-cadherin表达, 通过转染使C6细胞过表达T-cadherin可明显抑制C6细胞的增殖及转移, 表明T-cadherin分子具有抑癌基因功能[5]. 我们新近研究发现, T-cadherin基因表达缺失和下调与T-cadherin基因启动子甲基化、肝癌的恶性生物学特征密切相关, 约40%人肝癌组织中存在T-cadherin基因甲基化. 进一步研究发现5-Aza-CdR能使T-cadherin基因甲基化的人肝癌细胞株HepG2细胞恢复T-cadherin表达, 抑制HepG2细胞增殖[6]. 本研究进一步探讨5-Aza-CdR能否诱导裸鼠皮下HepG2种植瘤T-cadherin的表达, 并对裸鼠皮下HepG2种植瘤生长的抑制作用.
5-Aza-CdR购自美国Sigma药物公司; DMEM及胎牛血清购自Hyclone公司, HepG2细胞为华中科技大学同济医学院附属同济医院肝脏外科中心实验室提供; 裸鼠购自上海斯莱克实验动物中心, 为8 wk龄BALB/C型♂裸鼠, 饲养于华中科技大学同济医学院器官移植研究所SPF级动物房; T-cadherin兔抗人多克隆抗体购自Satan Cruz Biotechnology公司(编号: SC-7940), 兔/鼠通用型二抗(Dako公司)购自Gene公司; M-MLV逆转录酶及Taq酶为Fermentas公司产品, PCR引物由上海生物工程公司合成.
以含有100 mL/L胎牛血清的DMEM培养液培养HepG2细胞, 置于37℃、50 mL/L CO2培养箱中培养、传代.
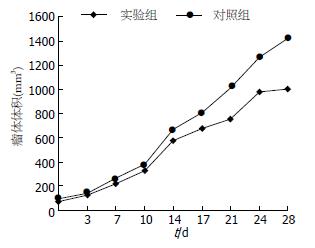
1.2.1 HepG2细胞裸鼠皮下种植瘤模型的建立及实验: 将培养至对数增殖期的HepG2细胞消化后、计数. 将含1×107个HepG2细胞悬浮于200 μL PBS液中, 注射于裸鼠侧腹皮下, 每只裸鼠注射左右两侧各一点, 共注射21只裸鼠. 注射后14 d, 裸鼠皮下形成肉眼可见的肿瘤(直径约0.3 cm)时, 将裸鼠随机分为实验组和对照组2组. 实验组11只, 对照组10只. 实验组裸鼠按1 mg/kg ip 5-Aza-CdR(溶解于200 μL PBS), 3 次/wk, 连续注射4 wk. 对照组裸鼠仅注射等量的PBS. 测量肿瘤长径(a), 短径(b), 计算其体积(V = ab2/2), 2 次/wk; 绘制肿瘤生长曲线. 在4 wk末处死裸鼠, 一部分肿瘤组织用40 g/L多聚甲醛固定, 石蜡包埋作免疫组织化学染色, 观察T-cadherin蛋白表达, 另一部分肿瘤组织用于提取总RNA, 分析T-cadherin基因mRNA表达.
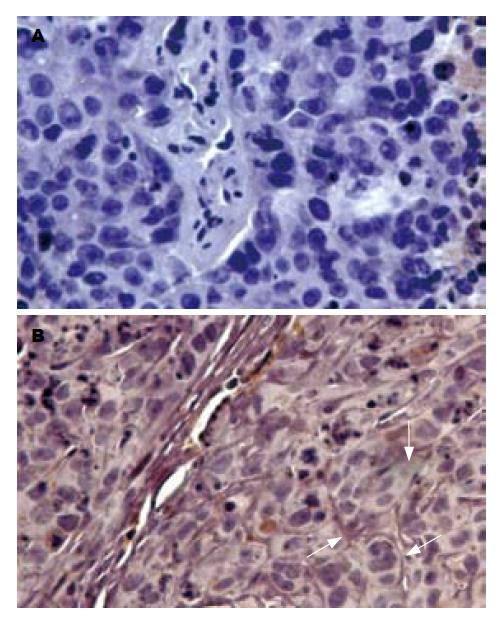
1.2.2 免疫组织化学: 取上述石蜡包埋的肿瘤组织, 连续切片, 每张4 μm厚. T-cadherin一抗用PBS以1:100稀释后4℃孵育过夜, PBS漂洗3次后, 加兔/鼠通用型二抗. 实验步骤按兔/鼠通用型免疫组化检测试剂盒的说明书进行. PBS代替一抗作为阴性对照, 细胞膜被染成棕黄色的细胞为T-cadherin表达阳性的细胞.
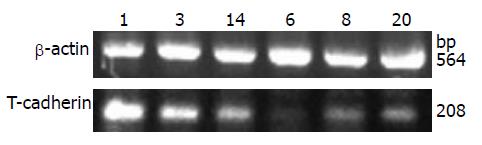
1.2.3 逆转录聚合酶链反应(RT-PCR): 用TRIzol试剂提取上述各裸鼠皮下种植瘤的总RNA(提取步骤按照TRIzol试剂的说明进行), 将其溶于DEPC处理水中, 用紫外光分光光度仪测浓度定量. 用2 μg的RNA作为模板进行逆转录反应, PCR反应的引物序列按照以前文章所述[2]. T-cadherin上游引物: 5'-TTCAGCAGAAAGTGTTCCATAT-3', 下游引物: 5'-GTGCATGGACGAACAGAGT-3', PCR产物长度为208 bp; 内参以β-actin作为对照, 上游引物: 5'-CTGGGACGACATGGAGAAAA-3', 下游引物: 5'-AAGGAAGGCTGGAAGAGTGC-3', PCR产物长度为564 bp. 循环过程包括: 起始, 94℃ 5 min; 变性, 94℃ 30 s; 退火, 54℃ 1 min; 延伸, 72℃ 1 min, 共35个循环; 最后72℃ 10 min. PCR产物经20 g/L琼脂糖凝胶电泳, 用AlphaEaseFC凝胶图像分析仪分析, 测定各条带的吸光度(A)值, 分别以T-cadherin/β-actin (A值的比值)表示T-cadherin mRNA的相对表达水平, 重复3次, 取平均值.
统计学处理 采用t检验. 数据均采用mean±SD表示, 以α = 0.05为检验水准, 采用SPSS13.0软件进行分析.
对照组裸鼠HepG2种植瘤细胞中T-cadherin mRNA呈低表达(T-cadherin基因PCR产物灰度值与β-actin PCR产物灰度值比值为0.256±0.079), 而注射5-Aza-CdR的裸鼠种植瘤细胞T-cadherin mRNA表达明显增强(T-cadherin基因PCR产物灰度值与β-actin PCR产物灰度值比值为0.545±0.119), 两者相比有统计学显著性差异(t = 6.613, P<0.05, 图1). 免疫组织化检测发现对照组裸鼠种植瘤细胞上均未见T-cadherin阳性着色, 而注射5-Aza-CdR后的裸鼠种植瘤细胞膜上可见明显棕黄色阳性着色(图2). 表明5-Aza-CdRip可恢复裸鼠HepG2种植瘤细胞的T-cadherin mRNA和蛋白表达.
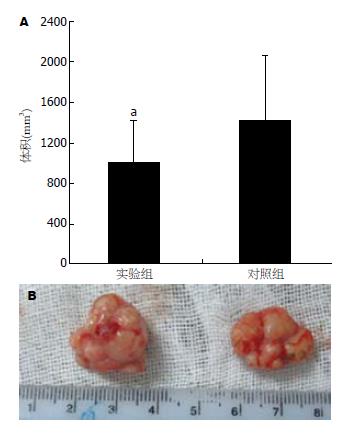
在给药期间, 共有2只裸鼠死亡, 实验组和对照组各死亡1只(分别于用药后第21天和23天死亡, 死亡原因可能与皮下种植瘤生长较快致恶病质有关). 用药后每周观察、测量肿瘤体积2次, 用药2 wk后即发现实验组裸鼠形成的肿瘤体积较对照组肿瘤体积小, 这种肿瘤体积的差异随给药时间延长而增大, 用药4 wk后肿瘤体积差异最明显(图3). 至4 wk后处死裸鼠时, 实验组肿瘤的体积为1005.78±419.69 mm3, 对照组为1423.78±645.54 mm3, 实验组肿瘤体积明显小于对照组肿瘤的体积(t = 2.337, P = 0.025, 图4).
肝细胞癌对放、化疗均不敏感, 对不能手术切除和切除后复发或转移的病例, 目前尚无有效的治疗手段. 深入研究肝细胞癌的发生、发展及转移的分子机制, 探索新的治疗靶点对肝癌治疗具有重要意义.
细胞黏附分子cadherin家族不仅介导细胞间的黏附[7], 而且还参与细胞间的信号传导机制[8-9]. T-cadherin是黏附分子家族较为特殊的一种类型, 因为缺失经典cadherin分子所具有的跨膜区而经糖基磷脂酰肌醇分子附着于细胞膜上[10], 故而命名truncated-cadherin(即T-cadherin, 又称CDH13或H-cadherin), 他主要介导同种细胞间的黏附和迁移[7,11]. 已有研究报道, T-cadherin在肺癌、胰腺癌等多种肿瘤中表达缺失[12-13]. 启动子甲基化是导致T-cadherin基因表达缺失或下调重要机制之一, 在卵巢癌[14]、胰腺癌[15-16]中已证实存在T-cadherin基因频繁甲基化. 在胰腺癌中, 约58%的T-cadherin的下调表达是由于启动子甲基化引起的[13]. 我们前期实验已经证实有40%人肝癌组织存在T-cadherin基因甲基化, 而癌周正常肝组织无T-cadherin基因甲基化. 肝癌中T-cadherin基因表达缺失和下调与T-cadherin基因启动子甲基化有关, 而T-cadherin基因表达缺失和下调是促进肝癌的恶性生物学特征进展的重要因素[6]. 由此可见, 通过对肝癌中甲基化的T-cadherin基因进行去甲基化处理, 可望恢复T-cadherin基因表达, 从而达到抑制肝癌发生、发展的目的.
去甲基化试剂5-Aza-CdR能通过共价健俘获甲基转移酶形成一个稳定的复合物, 使细胞在DNA复制过程中不能将S-腺苷-L-甲硫氨酸上的甲基基团转移至胞嘧啶上, 可有效消除细胞DNA甲基化的功能, 这可导致在DNA经过几个周期的复制后的去甲基化[17]. 5-Aza-CdR可使存在甲基化的多种肿瘤抑制基因去甲基化后表达, 达到抑制肿瘤生长的目的. Bender et al[3]发现用5-Aza-CdR处理过的T24膀胱癌细胞接种于裸鼠后形成的肿瘤较未处理的细胞接种的肿瘤明显减小. Lantry et al[4]发现5-Aza-CdR可以重新激活抑癌基因p16, 从而减少4-(甲基亚硝基)-3-(吡啶)-1-丁酮诱发的小鼠肺癌发生达23%. 临床应用5-Aza-CdR治疗肿瘤已见报道, 如利用5-Aza-CdR(通用名地西他滨, decitabine)治疗白血病及高危险性骨髓增生异常综合征等, 获得了较好疗效[18-19].
在体外研究发现人肝癌细胞株HepG2细胞存在T-cadherin基因启动子甲基化和T-cadherin基因低表达, 5-Aza-CdR作用HepG2细胞后能恢复T-cadherin表达, 并可抑制HepG2细胞增殖[6]. 本研究在裸鼠体内证实, 5-Aza-CdR对裸鼠体内的人肝癌HepG2细胞的生长具有明显的抑制作用. 其机制可能与5-Aza-CdR使甲基化的T-cadherin启动子去甲基化, 恢复T-cadherin基因表达有关. T-cadherin表达上调能可诱导p21表达导致细胞周期阻滞, 从而抑制肿瘤细胞增殖, 已在胶质母细胞瘤细胞中得以证实[5]. 然而, 5-Aza-CdR去甲基化作用并无特异性, 对多种甲基化的基因具有去甲基化作用, 恢复其基因功能. 在人肝癌中已证实存在甲基化的多种肿瘤抑制基因, 如E-cadherin基因[20]、与多数肿瘤发生密切相关的p15, p16基因[21]及p21基因[22]等均可能被去甲基化而恢复表达, 进而也会对肿瘤产生抑制作用. 因此, 对于5-Aza-CdR抑制裸鼠皮下HepG2种植瘤生长的分子机制有待于进一步深入研究.
本研究通过体内研究发现5-Aza-CdR对裸鼠体内的人肝癌HepG2细胞的生长具有明显的抑制作用. 为进一步利用甲基转移酶抑制剂5-Aza-CdR开展治疗肝癌的研究提供了新的思路.
近年来, 遗传表型改变在肝癌发病机制中的作用日益受到重视. 众多研究发现, 抑癌基因T-cadherin启动子内出现甲基化导致该基因表达下降或沉默是肝癌发病的重要途径之一. 已有体外实验证实5-杂氮-2'-脱氧胞苷能使T-cadherin基因甲基化的人肝癌细胞株HepG2细胞恢复T-cadherin表达, 抑制HepG2细胞增殖.
裘正军, 教授, 上海交通大学附属第一人民医院普通外科
近年来, 对抑癌基因失活与肿瘤发生之间关系的研究越来越受到人们的重视, 尤其是对抑癌基因启动子区CpG岛甲基化导致基因失活的研究已渐成为研究的热点.
Kantarjian利用5-杂氮-2'-脱氧胞苷(通用名地西他滨, decitabine)临床治疗白血病及高危险性骨髓增生异常综合征患者, 获得了较好疗效.
本文为进一步利用甲基转移酶抑制剂5-杂氮-2'-脱氧胞苷开展临床治疗肝癌的研究提供了实验依据以及可能的靶点.
本研究立题有一定新意, 方法成熟, 结论可信, 对进一步研究基因去甲基化治疗肿瘤提供了初步的实验依据, 具有一定学术价值.
编辑: 程剑侠 电编:何基才
| 1. | Sato N, Fukushima N, Maitra A, Matsubayashi H, Yeo CJ, Cameron JL, Hruban RH, Goggins M. Discovery of novel targets for aberrant methylation in pancreatic carcinoma using high-throughput microarrays. Cancer Res. 2003;63:3735-3742. [PubMed] |
| 2. | Toyooka KO, Toyooka S, Virmani AK, Sathyanarayana UG, Euhus DM, Gilcrease M, Minna JD, Gazdar AF. Loss of expression and aberrant methylation of the CDH13 (H-cadherin) gene in breast and lung carcinomas. Cancer Res. 2001;61:4556-4560. [PubMed] |
| 3. | Bender CM, Pao MM, Jones PA. Inhibition of DNA methylation by 5-aza-2'-deoxycytidine suppresses the growth of human tumor cell lines. Cancer Res. 1998;58:95-101. [PubMed] |
| 4. | Lantry LE, Zhang Z, Crist KA, Wang Y, Kelloff GJ, Lubet RA, You M. 5-Aza-2'-deoxycytidine is chemopreventive in a 4- (methyl-nitrosamino)-1-(3-pyridyl)-1-butanone-induced primary mouse lung tumor model. Carcinogenesis. 1999;20:343-346. [PubMed] [DOI] |
| 5. | Huang ZY, Wu Y, Hedrick N, Gutmann DH. T-cadherin-mediated cell growth regulation involves G2 phase arrest and requires p21(CIP1/WAF1) expression. Mol Cell Biol. 2003;23:566-578. [PubMed] [DOI] |
| 6. | Yan Q, Zhang ZF, Chen XP, Gutmann DH, Xiong M, Xiao ZY, Huang ZY. Reduced T-cadherin expression and promoter methylation are associated with the development and progression of hepatocellular carcinoma. Int J Oncol. 2008;32:1057-1063. [PubMed] |
| 8. | Levenberg S, Yarden A, Kam Z, Geiger B. p27 is involved in N-cadherin-mediated contact inhibition of cell growth and S-phase entry. Oncogene. 1999;18:869-876. [PubMed] [DOI] |
| 9. | Ivanov DB, Philippova MP, Tkachuk VA. Structure and functions of classical cadherins. Biochemistry (Mosc). 2001;66:1174-1186. [PubMed] [DOI] |
| 10. | Angst BD, Marcozzi C, Magee AI. The cadherin superfamily: diversity in form and function. J Cell Sci. 2001;114:629-641. [PubMed] |
| 11. | Philippova M, Ivanov D, Tkachuk V, Erne P, Resink TJ. Polarisation of T-cadherin to the leading edge of migrating vascular cells in vitro: a function in vascular cell motility? Histochem Cell Biol. 2003;120:353-360. [PubMed] [DOI] |
| 12. | Sato M, Mori Y, Sakurada A, Fujimura S, Horii A. The H-cadherin (CDH13) gene is inactivated in human lung cancer. Hum Genet. 1998;103:96-101. [PubMed] [DOI] |
| 13. | Sakai M, Hibi K, Koshikawa K, Inoue S, Takeda S, Kaneko T, Nakao A. Frequent promoter methylation and gene silencing of CDH13 in pancreatic cancer. Cancer Sci. 2004;95:588-591. [PubMed] [DOI] |
| 14. | Merlo A, Herman JG, Mao L, Lee DJ, Gabrielson E, Burger PC, Baylin SB, Sidransky D. 5' CpG island methylation is associated with transcriptional silencing of the tumour suppressor p16/CDKN2/MTS1 in human cancers. Nat Med. 1995;1:686-692. [PubMed] [DOI] |
| 15. | Dammann R, Schagdarsurengin U, Liu L, Otto N, Gimm O, Dralle H, Boehm BO, Pfeifer GP, Hoang-Vu C. Frequent RASSF1A promoter hypermethylation and K-ras mutations in pancreatic carcinoma. Oncogene. 2003;22:3806-3812. [PubMed] [DOI] |
| 16. | Fukushima N, Sato N, Ueki T, Rosty C, Walter KM, Wilentz RE, Yeo CJ, Hruban RH, Goggins M. Aberrant methylation of preproenkephalin and p16 genes in pancreatic intraepithelial neoplasia and pancreatic ductal adenocarcinoma. Am J Pathol. 2002;160:1573-1581. [PubMed] |
| 17. | Santini V, Kantarjian HM, Issa JP. Changes in DNA methylation in neoplasia: pathophysiology and therapeutic implications. Ann Intern Med. 2001;134:573-586. [PubMed] |
| 18. | Wijermans PW, Krulder JW, Huijgens PC, Neve P. Continuous infusion of low-dose 5-Aza-2'-deoxycytidine in elderly patients with high-risk myelodysplastic syndrome. Leukemia. 1997;11:1-5. [PubMed] [DOI] |
| 19. | Kantarjian H, Oki Y, Garcia-Manero G, Huang X, O'Brien S, Cortes J, Faderl S, Bueso-Ramos C, Ravandi F, Estrov Z. Results of a randomized study of 3 schedules of low-dose decitabine in higher-risk myelodysplastic syndrome and chronic myelomonocytic leukemia. Blood. 2007;109:52-57. [PubMed] [DOI] |
| 20. | Kanai Y, Ushijima S, Hui AM, Ochiai A, Tsuda H, Sakamoto M, Hirohashi S. The E-cadherin gene is silenced by CpG methylation in human hepatocellular carcinomas. Int J Cancer. 1997;71:355-359. [PubMed] [DOI] |
| 21. | Lee YY, Kang SH, Seo JY, Jung CW, Lee KU, Choe KJ, Kim BK, Kim NK, Koeffler HP, Bang YJ. Alterations of p16INK4A and p15INK4B genes in gastric carcinomas. Cancer. 1997;80:1889-1896. [PubMed] [DOI] |
| 22. | Vogt M, Haggblom C, Yeargin J, Christiansen-Weber T, Haas M. Independent induction of senescence by p16INK4a and p21CIP1 in spontaneously immortalized human fibroblasts. Cell Growth Differ. 1998;9:139-146. [PubMed] |