修回日期: 2008-03-25
接受日期: 2008-05-20
在线出版日期: 2008-05-28
目的: 研究脐带间充质干细胞(umbilical cord-mesenchymal stem cells, UC-MSCs)生物学的特性及向肝细胞分化的可能性.
方法: 从脐带中分离间充质干细胞, 体外行传代培养, 检测脐带间充质干细胞表面免疫标志、细胞周期和生长活性等, 利用肝细胞生长因子、成纤维生长因子4和抑瘤素等细胞因子诱导脐带间充质干细胞向肝细胞分化, 用免疫细胞方法对诱导和未诱导的细胞进行免疫学检测, 糖原染色进行功能鉴定.
结果: 从人脐带中可分离到贴壁生长的间充质干细胞, 细胞形态类似成纤维细胞,可在体外进行长期稳定培养; CD29、CD105和Vimentin表达阳性, 基本不表达CD34、CD31, 经加入细胞因子可成功将间充质干细胞向肝细胞诱导分化, 分化的细胞表达肝细胞表面标志物ALB、AFP、CK18和CK19, 糖原染色呈现阳性.
结论: 人脐带中可成功分离到间充质干细胞, 细胞可实现体外长期培养, 表达脐带间充质干细胞的表面标志, 在体外脐带间充质干细胞诱导分化为肝细胞, 有望成为细胞替代治疗的理想来源之一.
引文著录: 闫俊卿, 韩涛, 朱争艳. 人脐带间充质干细胞生物学特性及向类肝细胞的分化. 世界华人消化杂志 2008; 16(15): 1639-1644
Revised: March 25, 2008
Accepted: May 20, 2008
Published online: May 28, 2008
AIM: To investigate the biological characteristics of human umbilical cord-derived mesenchymal stem cells (MSCs), and to study the possibility of their differentiation into hepatocyte-like cells.
METHODS: MSCs were isolated from human umbilical cord; after serial subcultivation in vitro, surface immunological markers in MSCs were detected by immunocytochemical staining and flow cytometry. cell cycle and growth activity were also detected. Hepatocyte growth factor (HGF), fibroblast growth factor-4 (FGF-4), oncostatin M (OM) were adopted to effectively induce hepatic differentiation, and specific surface phenotype of liver cells was detected by immunocytochemical staining. In vitro functions of the induced and uninduced cells were identified by glycogen staining.
RESULTS: MSCs were isolated from human umbilical cord sucessfully, presenting fibroblastic morphology and long-term stability. These cells were positive for CD29, CD105, and Vimentin, but were negative for CD34 and CD31. MSCs could be induced to differentiate into hepatocyte-1ike cells that were positive for albumin, alpha fetoprotein, cytokeratin 18, cytokeratin 19 and glycogen staining.
CONCLUSION: Human umbilical cord-derived MSCs are able to differentiate into functional hepatocyte-like cells, and may serve as a cell source for tissue engineering and cell therapy of hepatic diseases.
- Citation: Yan JQ, Han T, Zhu ZY. Biological characteristics and differentiation into hepatocyte-like cells of human umbilical cord-derived mesenchymal stem cells. Shijie Huaren Xiaohua Zazhi 2008; 16(15): 1639-1644
- URL: https://www.wjgnet.com/1009-3079/full/v16/i15/1639.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v16.i15.1639
间充质干细胞(mesenchymal stem cells, MSCs)是一类具有显著的自我更新和多向分化能力的细胞, 他来源丰富, 主要有骨髓、脐血、脐带、全身结缔组织和器官间质[1-3]. 目前骨髓是实验和临床研究中MSCs的主要来源, 但骨髓存在有自身局限性, 脐血中能否获得MSCs仍存在有争议[4-5]. 已有研究报道脐带中富含MSCs, 易于获得, 分离成功率高, 具有多向分化的能力, 因此选择脐带作为研究对象. 本实验旨在通过研究其生物学特性及向肝细胞分化的能力, 为人工肝、肝细胞移植等提供理想的种子细胞.
脐带取自天津市第三中心医院住院的健康足月产妇, 均征得父母授权同意及伦理委员会批准. DMEM/F12培养基、胎牛血清(FBS)、Ⅳ型胶原酶均为美国Gibco公司产品; 肝细胞生长因子(HGF)、成纤维生长因子4(FGF4)、抑瘤素(OSM)购自美国Properto; ITS(25 g/L胰岛素、25 g/L转铁蛋白、25 mg/L亚硒酸钠)、胰酶为美国Sigma公司产品; 免疫组化用mAb和SABC试剂盒为中山金桥公司产品; 荧光标记小鼠抗人抗体: CD29-FITC、CD34-PE、CD90-PE、CD105-FITC购自晶美生物有限公司; 红细胞裂解液;细胞培养基: DMEM/F 12+50 mL/L FBS+100 kU/L青霉素+100 kU/L链霉素.
1.2.1 细胞培养: 脐带自手术台取下后, 浸入PBS内, 4℃保存, 在操净台内取出脐带, 用PBS冲洗净脐动脉和脐静脉内的血液, 脐带剪碎后, 置于恒温震荡仪内, 加入Ⅳ型胶原酶(1 g/L)60 min, 胰酶(1.25 g/L)30 min, 经完全消化后, 滤网过滤收集细胞, 加入适量红细胞裂解液进一步去除红细胞. 细胞培养基重悬细胞, 调整细胞密度至5×106/L, 接种于T25培养瓶内, 孵箱内培养, 5 d后换液, 每3 d换液1次, 待细胞生长至80%-90%融合后进行传代培养.
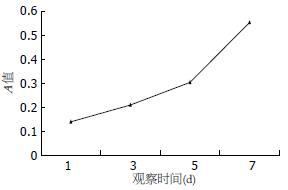
1.2.2 细胞生长活性测定: 取脐带MSCs, 调整细胞密度至5×103/L, 接种于96孔板内, 于接种后1、3、5、7 d利用MTT法进行检测; 每组6孔, 同时以无细胞培养液作为空白对照, 于490 nm处分光光度计检测各组细胞的A值, 取均值绘制生长曲线.
1.2.3 细胞周期分析: 取对数生长期细胞, 制备单细胞悬液, 酒精固定48 h. 加入1 mL PI染液4℃避光染色30 min. 流式细胞仪(美国Beckman公司)检测, MultiCycle软件对该细胞周期DNA含量进行分析, 确定细胞周期分布.
1.2.4 免疫细胞化学检测: 取培养脐带MSCs, 制备细胞涂片, 用CD105、Vimentin、CD34 、CD31 mAb对脐带MSCs进行免疫细胞化学染色, 按SABC试剂盒要求操作, 置显微镜下观察染色情况, 结果进行拍照.
1.2.5 流式细胞仪检测: 取脐带MSCs经胰酶消化后, 充分吹打制备单细胞悬液, 加入待检测指标CD29-FITC、CD34-PE、CD105-FITC, 流式细胞仪进行检测.
1.2.6 诱导分化: 取第9代细胞接种于12孔板内, 细胞浓度调整为5×107/L, 设置对照组和诱导组, 无血清培养1 d后, 加入DMEM培养基、HGF(20 μg/L)、FGF4(10 μg/L)、ITS(50 g/L)、地塞米松(0.5 μmol)、烟碱(0.61 g/L)培养10 d后, 加入DMEM培养基、OSM(50 mg/L)、地塞米松(0.5 μmol)、ITS(50 g/L)培养18 d, 共培养28 d, 分别于1、2、3和4 wk取细胞进行相关实验.
1.2.7 肝细胞的免疫细胞化学染色鉴定: 分别于1、2、3和4 wk收集细胞进行检测, 观察不同时间点细胞形态的变化, 细胞化学染色测定肝细胞特异表面标志物ALB、AFP、CK18和CK19(方法同上).
1.2.8 糖原染色: 取不同时间点对照组和诱导组细胞, 中性福尔马林固定10 min, 10 g/L碘酸水溶液5-10 min, 蒸馏水洗多次后晾干, Schiff液10-15 min, 蒸馏水洗5 min, 苏木素复染细胞核1-3 min, 蒸馏水洗5 min, 脱水, 透明, 封片.
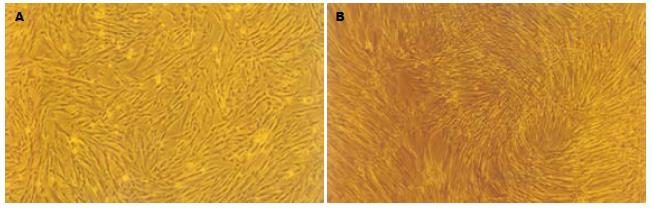
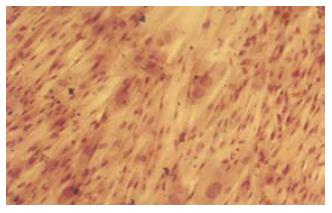
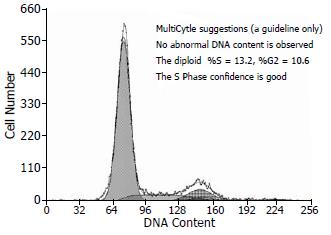
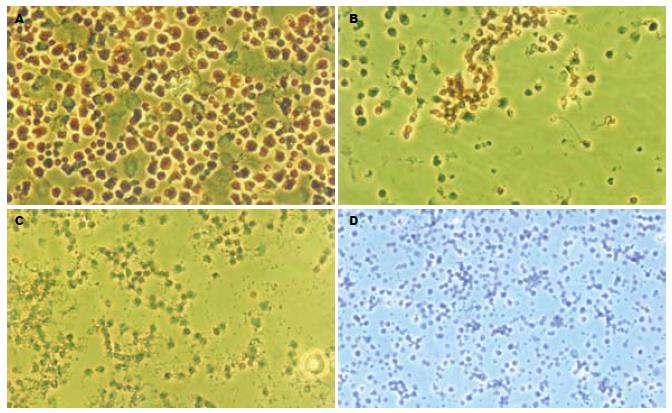
人脐带MSCs经剪碎后加入Ⅳ型胶原酶及胰酶, 可有效地分离到MSCs, 细胞呈贴壁生长特性, 形态为长梭形, 类似于成纤维细胞, 漩涡样生长. 细胞大小不一, 经10-15 d培养后进行传代培养, 细胞增殖速度快, 约每周传代1次, 传代次数已达到20次以上(图1), HE染色可见细胞体积较大, 核浆比例大(图2), 细胞生长80%以上处于G0/G1期(图3), MTT检测可见细胞生长呈上升趋势, 具有良好的长活性(图4).
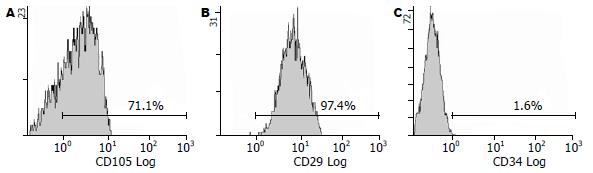
细胞表达CD29、CD105和Vimentin, 基本不表达造血细胞标志CD34和内皮细胞标志CD31. 流式细胞仪检测示表达CD29、CD105和CD90, 不表达CD34与免疫细胞化学检测结果一致(图5-6).
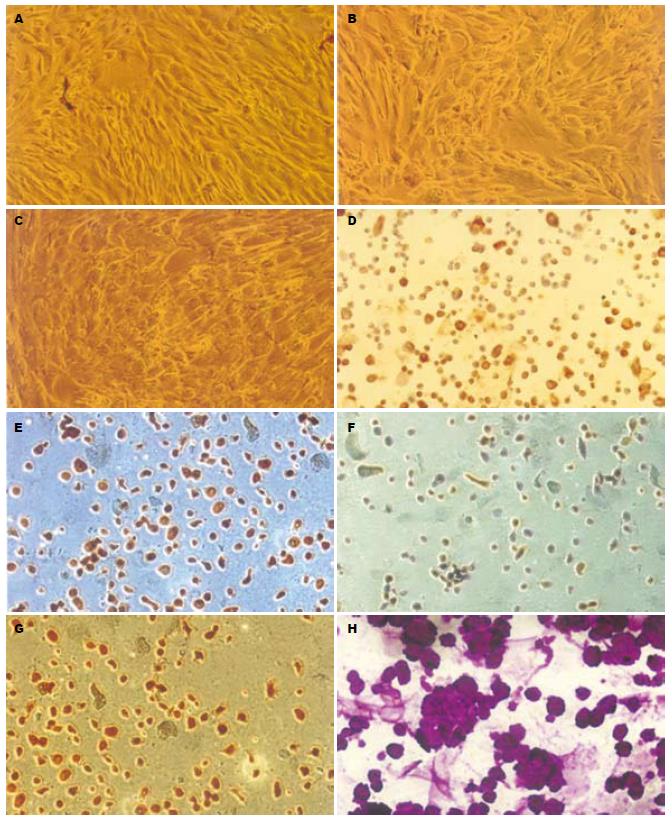
通过实验分别于光镜下观察诱导前和诱导后1、2、3、4 wk细胞形态的变化, 诱导前细胞具有MSCs的细胞形态, 呈长梭形, 不规则形, 贴壁生长. 在诱导后1 wk可观察到少部分细胞突起逐渐消失, 逐渐变圆, 随着诱导时间的延长, 圆形细胞逐渐增多, 细胞形态似肝细胞(图7A-C). 于不同时间点收集诱导后的细胞, 行免疫细胞化学染色观察, 在诱导1 wk时细胞开始表达肝细胞表面标志物AFP、CK18和CK19, 不表达ALB; 在诱导3 wk时, 开始表达成熟肝细胞标志ALB, 同时观察到AFP、CK18和CK19的表达下降. 在4 wk时, ALB表达增强(图7D-G). 对实验组和对照组细胞进行糖原PAS染色, 4 wk时对照组细胞糖原染色阳性, 说明经诱导后的细胞具有肝细胞功能特性(图7H).
MSCs是中胚层来源的一类具有多向分化能力的干细胞[6-7], 相比较各种来源的MSCs可发现脐带更具优势, 他易于获得, 取材方便, 排除了伦理道德方面的限制, 可用于广泛研究. 经多数研究证实脐带来源的MSCs与骨髓来源的MSCs在生物学特性方面极为相似, 且可进一步分化为心肌细胞、脂肪细胞和神经细胞等[8-14], 关于他向肝细胞分化能力的报道尚少.
MSCs缺乏特异的细胞表面标志物, 表达CD29、CD44、CD54、CD105、CD73和CD166; 不表达CD3、CD34、CD45、CD117、CD133、CD31和HLA-DR等[3,15-16]. 因此不宜用流式、免疫磁珠等方法分离. 本实验通过其贴壁生长的特性进行纯化, 经免疫细胞化学和流式细胞仪检测得到细胞强表达CD29、CD105和Vimentin;不表达CD34和CD31. 与文献报道相一致[17-19].
MSCs向肝细胞的诱导分化突破了胚层的限制, 实现了跨胚层分化, 是一个复杂的变化过程, 需要模拟体内肝损伤后的肝细胞再生微环境及众多的细胞因子参与才可实现, 目前体外实验中用于肝细胞诱导的主要有HGF、FGF4、EGF、OSM、ITS和地塞米松等, 其中肝细胞生长因子可刺激肝细胞的增殖并参于肝脏的再生, 是调节肝脏生长和功能的关键因子. 通过参考众多文献及进行多次预实验后, 我们将文献所报道的诱导方法进行优化改进, 调整诱导因子的用量及作用时间, 实现MSCs向肝细胞的诱导[20-22].
实验中我们进行免疫细胞化学检测了肝细胞特异标志AFP、ALB、CK18和CK19, 结果发现在诱导的过程中细胞可表达上述指标, 本实验中诱导组1 wk开始表达AFP, 到4 wk时已基本不表AFP, 而ALB呈现阳性表达, CK18、CK19在2 wk开始有表达, 初步分析得出MSCs向肝细胞分化是一个逐渐变化的过程, 由未成熟到成熟的过程. 肝脏是唯一能够生成和储存糖原的器官, 成熟的肝细胞具有生成糖原的能力, 于4 wk糖原染色呈现阳性, 说明诱导后细胞初步具有成熟肝细胞的特性[23-27]. 这些研究结果与文献报道相一致.
因此通过实验证明人脐带MSCs在一定条件下可转化为类肝细胞, 然而这仅是一个最基本的研究, 我们对这种跨胚层的分化过程认识的远远不够, 分化是如何启动、调控、终止仍是一团迷雾, 所以欲使脐带MSCs成为细胞移植治疗及组织工程的理想种子细胞仍有待研究[28-29].
目前肝细胞移植用于治疗终末期肝病被认为是一种较为有效的治疗手段之一, 肝细胞的来源成为近年来的研究热点, UC-MSCs易于获得, 能够实现体外大量扩增, 具有多向分化能力, 是一种较为理想的细胞来源.
任粉玉, 副教授, 延边大学附属医院消化内科.
MSCs的来源较为广泛, 究竟何种来源的MSCs能够成为细胞治疗的理想种子及其在多向分化过程中的调控机制成为目前的研究热点、重点和亟待解决的问题.
研究发现脐带中MSCs分离成功率高, 在体外能够大量扩增, 因而越来越受到大家的关注.
本实验在总结以往研究的基础上, 进一步优化诱导方案, 实现了UC-MSCs向肝细胞的诱导分化.
本实验通过研究UC-MSCs的生物学特性及向肝细胞的诱导分化, 为间充质干细胞提供了新的来源, 同时为肝病的治疗带来新的思路.
本研究内容新颖, 设计基本合理, 结果可信, 具有较好的学术价值.
编辑: 李军亮 电编:何基才
| 1. | Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143-147. [PubMed] [DOI] |
| 2. | Mareschi K, Biasin E, Piacibello W, Aglietta M, Madon E, Fagioli F. Isolation of human mesenchymal stem cells: bone marrow versus umbilical cord blood. Haematologica. 2001;86:1099-1100. [PubMed] |
| 3. | Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25:2739-2749. [PubMed] [DOI] |
| 4. | Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. 2000;109:235-242. [PubMed] [DOI] |
| 5. | Wexler SA, Donaldson C, Denning-Kendall P, Rice C, Bradley B, Hows JM. Adult bone marrow is a rich source of human mesenchymal 'stem' cells but umbilical cord and mobilized adult blood are not. Br J Haematol. 2003;121:368-374. [PubMed] [DOI] |
| 6. | D'Ippolito G, Schiller PC, Ricordi C, Roos BA, Howard GA. Age-related osteogenic potential of mesenchymal stromal stem cells from human vertebral bone marrow. J Bone Miner Res. 1999;14:1115-1122. [PubMed] [DOI] |
| 7. | Sudo K, Kanno M, Miharada K, Ogawa S, Hiroyama T, Saijo K, Nakamura Y. Mesenchymal progenitors able to differentiate into osteogenic, chondrogenic, and/or adipogenic cells in vitro are present in most primary fibroblast-like cell populations. Stem Cells. 2007;25:1610-1617. [PubMed] [DOI] |
| 8. | Wang HS, Hung SC, Peng ST, Huang CC, Wei HM, Guo YJ, Fu YS, Lai MC, Chen CC. Mesenchymal stem cells in the Wharton's jelly of the human umbilical cord. Stem Cells. 2004;22:1330-1337. [PubMed] [DOI] |
| 9. | Lu LL, Liu YJ, Yang SG, Zhao QJ, Wang X, Gong W, Han ZB, Xu ZS, Lu YX, Liu D. Isolation and characterization of human umbilical cord mesenchymal stem cells with hematopoiesis-supportive function and other potentials. Haematologica. 2006;91:1017-1026. [PubMed] |
| 10. | Friedman R, Betancur M, Boissel L, Tuncer H, Cetrulo C, Klingemann H. Umbilical cord mesenchymal stem cells: adjuvants for human cell transplantation. Biol Blood Marrow Transplant. 2007;13:1477-1486. [PubMed] [DOI] |
| 11. | Romanov YA, Svintsitskaya VA, Smirnov VN. Searching for alternative sources of postnatal human mesenchymal stem cells: candidate MSC-like cells from umbilical cord. Stem Cells. 2003;21:105-110. [PubMed] [DOI] |
| 12. | Wagner W, Wein F, Seckinger A, Frankhauser M, Wirkner U, Krause U, Blake J, Schwager C, Eckstein V, Ansorge W. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp Hematol. 2005;33:1402-1416. [PubMed] [DOI] |
| 13. | Secco M, Zucconi E, Vieira NM, Fogaça LL, Cerqueira A, Carvalho MD, Jazedje T, Okamoto OK, Muotri AR, Zatz M. Multipotent stem cells from umbilical cord: cord is richer than blood! Stem Cells. 2008;26:146-150. [PubMed] [DOI] |
| 14. | Hardy SA, Maltman DJ, Przyborski SA. Mesenchymal stem cells as mediators of neural differentiation. Curr Stem Cell Res Ther. 2008;3:43-52. [PubMed] [DOI] |
| 16. | Fong CY, Richards M, Manasi N, Biswas A, Bongso A. Comparative growth behaviour and characterization of stem cells from human Wharton's jelly. Reprod Biomed Online. 2007;15:708-718. [PubMed] |
| 17. | Titorencu I, Jinga VV, Constantinescu E, Gafencu AV, Ciohodaru C, Manolescu I, Zaharia C, Simionescu M. Proliferation, differentiation and characterization of osteoblasts from human BM mesenchymal cells. Cytotherapy. 2007;9:682-696. [PubMed] [DOI] |
| 18. | Schrepfer S, Deuse T, Lange C, Katzenberg R, Reichenspurner H, Robbins RC, Pelletier MP. Simplified protocol to isolate, purify, and culture expand mesenchymal stem cells. Stem Cells Dev. 2007;16:105-107. [PubMed] [DOI] |
| 19. | Beyer Nardi N, da Silva Meirelles L. Mesenchymal stem cells: isolation, in vitro expansion and characterization. Handb Exp Pharmacol. 2006;249-282. [PubMed] |
| 20. | Lee KD, Kuo TK, Whang-Peng J, Chung YF, Lin CT, Chou SH, Chen JR, Chen YP, Lee OK. In vitro hepatic differentiation of human mesenchymal stem cells. Hepatology. 2004;40:1275-1284. [PubMed] [DOI] |
| 21. | Seo MJ, Suh SY, Bae YC, Jung JS. Differentiation of human adipose stromal cells into hepatic lineage in vitro and in vivo. Biochem Biophys Res Commun. 2005;328:258-264. [PubMed] [DOI] |
| 22. | Chen Y, Dong XJ, Zhang GR, Shao JZ, Xiang LX. In vitro differentiation of mouse bone marrow stromal stem cells into hepatocytes induced by conditioned culture medium of hepatocytes. J Cell Biochem. 2007;102:52-63. [PubMed] [DOI] |
| 23. | Hong SH, Gang EJ, Jeong JA, Ahn C, Hwang SH, Yang IH, Park HK, Han H, Kim H. In vitro differentiation of human umbilical cord blood-derived mesenchymal stem cells into hepatocyte-like cells. Biochem Biophys Res Commun. 2005;330:1153-1161. [PubMed] [DOI] |
| 24. | Kang XQ, Zang WJ, Bao LJ, Li DL, Song TS, Xu XL, Yu XJ. Fibroblast growth factor-4 and hepatocyte growth factor induce differentiation of human umbilical cord blood-derived mesenchymal stem cells into hepatocytes. World J Gastroenterol. 2005;11:7461-7465. [PubMed] |
| 25. | Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology. 2006;43:S45-S53. [PubMed] [DOI] |
| 26. | Campard D, Lysy PA, Najimi M, Sokal EM. Native umbilical cord matrix stem cells express hepatic markers and differentiate into hepatocyte-like cells. Gastroenterology. 2008;134:833-848. [PubMed] [DOI] |
| 27. | Yamamoto Y, Banas A, Murata S, Ishikawa M, Lim CR, Teratani T, Hatada I, Matsubara K, Kato T, Ochiya T. A comparative analysis of the transcriptome and signal pathways in hepatic differentiation of human adipose mesenchymal stem cells. FEBS J. 2008;275:1260-1273. [PubMed] [DOI] |
| 28. | Salgado AJ, Oliveira JT, Pedro AJ, Reis RL. Adult stem cells in bone and cartilage tissue engineering. Curr Stem Cell Res Ther. 2006;1:345-364. [PubMed] |
| 29. | Yoshikawa T, Mitsuno H, Nonaka I, Sen Y, Kawanishi K, Inada Y, Takakura Y, Okuchi K, Nonomura A. Wound therapy by marrow mesenchymal cell transplantation. Plast Reconstr Surg. 2008;121:860-877. [PubMed] |