修回日期: 2008-03-12
接受日期: 2008-04-21
在线出版日期: 2008-04-28
目的: 探讨外源性干细胞因子(stem cell factor, SCF)能否改善糖尿病(diabetes mellitus, DM)小鼠结肠Cajal间质细胞(interstitial cells of Cajal, ICC)的异常病变.
方法: DM小鼠一次性ip链脲佐菌素(STZ, 150 mg/kg)造模, 将♂C57/BL6小鼠分为正常对照组(control组)、糖尿病组(DM组)、糖尿病+外源性SCF组(DM+SCF组); DM+SCF组ip SCF 0.2 µg/(kg•d), control组和DM组每天ip等量的磷酸盐缓冲液(pH = 7.4), 干预6 wk后处死所有小鼠, 以Western blot检测远端结肠组织中SCF的表达情况, 以免疫组化、透射电镜和Western blot观察远端结肠ICC的变化.
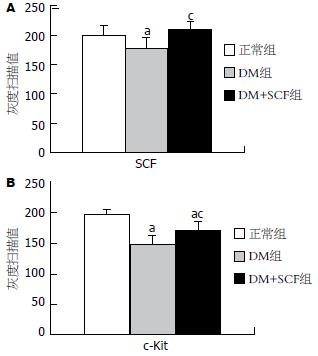
结果: DM小鼠远端结肠组织中SCF水平明显降低(178.97±13.51 vs 200.25±16.48, P<0.05), 且伴结肠组织中ICC数量减少(72±10 vs 102±12, P<0.05)、超微结构严重破坏. DM鼠给予外源性SCF干预后, 结肠组织中SCF表达(210.14±11.8)上调(P<0.05), 且ICC的数量(87±10, P<0.05)和超微结构显著改善.
结论: 外源性SCF对糖尿病相关的结肠ICC异常病变有一定改善或逆转作用.
引文著录: 徐丽明, 林琳, 汤玉蓉, 张红杰, 李学良. 干细胞因子对糖尿病小鼠结肠Cajal间质细胞的干预效应. 世界华人消化杂志 2008; 16(12): 1294-1298
Revised: March 12, 2008
Accepted: April 21, 2008
Published online: April 28, 2008
AIM: To investigated whether exogenous stem cell factor (SCF) can improve the diabetes-associated depletion of interstitial cells of Cajal (ICC) in mice with diabetes mellitus (DM).
METHODS: DM mice were intraperitoneally injected with streptozocin (STZ) to induce an experimental model. Male C57/BL6 mice were randomly divided into control group, DM group and DM + SCF group. The mice in DM + SCF group were given exogenous SCF (0.2 µg/kg per day, ip) for 6 wk, and the mice in control group and DM group were given the same amount of phosphate buffer (pH = 7.4). All the mice were sacrificed after 6 wk. ICC changes in the distal colon were assessed by immunohistochemistry, transmission electron microscopy and Western blot, and SCF expression in the distal colon was analyzed by Western blot.
RESULTS: The expression of SCF in the distal colon was significantly reduced in DM group as compared with that in the control group (178.97 ± 13.51 vs 200.25 ± 16.48, P < 0.05), accompanied with the depletion (72 ± 10 vs 102 ± 12, P < 0.05) and microscopic lesions of ICC in the distal colon. The expression of SCF in the distal colon was increased in DM + SCF group (210.14 ± 11.8, P < 0.05), along with the dramatic improvement of ICC quantity (87 ± 10, P < 0.05) and ultrastructure in the distal colon as compared with those in DM group.
CONCLUSION: Exogenous SCF may improve the DM-associated depletion of colon ICC.
- Citation: Xu LM, Lin L, Tang YR, Zhang HJ, Li XL. Effect of stem cell factor on colon interstitial cells of Cajal in murine with diabetes mellitus. Shijie Huaren Xiaohua Zazhi 2008; 16(12): 1294-1298
- URL: https://www.wjgnet.com/1009-3079/full/v16/i12/1294.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v16.i12.1294
糖尿病(diabetes mellitus, DM)患者中, 胃肠动力障碍的发病率为25%-76%[1-2], 结肠动力障碍是DM常见并发症之一, 临床上可有便秘与腹泻等症状, 其中便秘在1型DM患者中发病率很高[3], 也是2型DM最常见的胃肠道症状[4], 严重影响患者的生活质量与血糖控制. Cajal间质细胞(interstitial cells of Cajal, ICC)是胃肠道起搏细胞, 且介导神经递质的产生和作用[5-6], 其数量和结构改变可能是DM结肠动力障碍重要原因[7-9]. ICC特异性表达的酪氨酸激酶受体c-Kit, 其配体干细胞因子[10](stem cell factor, SCF)已被证实对ICC具有重要调控作用[11-12]. 本研究将初步探讨外源性SCF对DM小鼠结肠ICC的作用或影响.
4-6 wk♂C57/BL6小鼠(上海斯莱克公司), 链脲佐菌素(streptozocin, STZ, Sigma, USA), SCF抗体(R&D, UK), c-Kit抗体(Santa Cruz, USA), 二抗(Santa Cruz, USA), philips EM-400透射电镜等.
1.2.1 建立DM小鼠模型、分组及干预: DM小鼠予一次性ip STZ(150 mg/kg)造模[13-14], 72 h后及1 wk后尾静脉采血, 两次血糖均≥12 mmol/L者为DM小鼠模型建立, 不符合条件者予以剔除. 所有实验小鼠分为正常对照组(control组, n = 6)、糖尿病组(DM组, n = 6)和糖尿病+外源性SCF干预组(DM+SCF组, n = 6). 干预(共6 wk): DM+SCF组给予SCF ip[0.2 µg/(kg•d)], control组和DM组每天ip等量的磷酸盐缓冲液(pH = 7.4), 6 wk结束后, 停止干预3 d后处死各组小鼠. 取小鼠距肛门1 cm左右的远端结肠组织约2 cm.
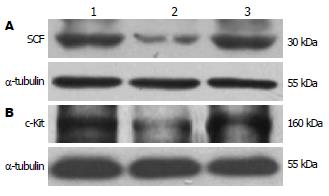
1.2.2 结肠组织SCF及c-Kit的表达(Western blot法): 取约0.5 g远端结肠研磨提取蛋白, BCA法测定蛋白浓度. 按50 µg蛋白/泳道加样, 恒流40 mA电泳, 恒压100 V转膜2 h, 50 mL/L牛奶封闭1.5 h. 检测SCF时, 加入SCF一抗(1:500), 37℃孵育1 h, 4℃过夜, 加入二抗(1:2000), 37℃孵育2 h, 曝光、显影. 检测c-Kit时, 一抗浓度为1:200, 二抗浓度为1:1500, 其余条件同前.
1.2.3 结肠组织c-Kit的表达(免疫组化, SP法): 取远端结肠约0.5 cm, 常规固定、包埋、切片, 脱蜡至水、抗原修复. 加c-Kit一抗(1:100)50 μL, 37℃孵育1 h后4℃过夜, 加生物素标记的二抗及链霉素抗生物素蛋白-过氧化酶各50 μL, 室温孵育各15 min, DAB显色, 复染、脱水、封片. 结果判断: 显微镜下胞质出现棕黄色片状或颗粒状物为阳性.
1.2.4 ICC超微结构观察(电镜): 取远端结肠约0.5 cm, 放入戊二醛固定, 经前固定、后固定、块染、脱水、浸渍后包埋、聚合, 半薄切片定位观察, 确定组织分层后, LKB-Ⅱ超薄切片机切片, 染色后透射电镜观察.
统计学处理 所有数据录入SPSS10.0软件包分析, 以mean±SD表示, 采用方差分析和成组t检验, P<0.05为有统计学差异.
干预6 wk后, DM组远端结肠中SCF和c-Kit蛋白分别较正常对照组显著降低(178.97±13.51 vs 200.25±16.48, 146.24±16.64 vs 196.73±8.48, P<0.05), DM+SCF组较DM组显著增加(210.14±11.8 vs 178.97±13.51, 169.36±15.8 vs 146.24±16.64, P<0.05). DM+SCF组与正常对照组中SCF蛋白表达无统计学差异(P>0.05), 而c-Kit蛋白有统计学差异 (P<0.05, 图1-2).
干预6 wk后, DM组远端结肠c-Kit阳性细胞数较正常对照组明显减少(72±10 vs 102±12/5个高倍镜视野, P<0.05), DM+SCF组较DM组显著增加(87±10 vs 72±10/5个高倍镜视野, P<0.05), 但尚未达到正常对照组水平(87±10 vs 102±12/5个高倍镜视野, P<0.05, 图3).
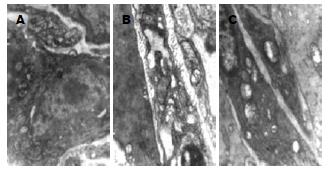
正常对照组小鼠远端结肠中ICC核膜完整, 胞质内富含细胞器, 如内质网、线粒体等, 细胞器结构清晰, 发育良好; ICC与周围的ICC、神经纤维以及平滑肌细胞间形成紧密连接. DM组小鼠远端结肠ICC出现基膜溶解, 细胞器破坏、数量减少, 内质网扩张, 线粒体肿胀甚至空泡样变性; ICC与周围细胞之间的紧密连接也被破坏. DM+SCF组结肠ICC的结构病变较DM组有所改善(图4).
DM结肠动力障碍的病理生理特点是: 结肠张力和收缩力低下、蠕动减慢、排空延迟[15], 其机制尚不完全清楚, 肠道ICC病变是重要原因之一[7], 临床缺乏有效的治疗手段. ICC是分布在胃肠神经末梢与平滑肌细胞之间的一类特殊细胞群, 他不仅是胃肠道起搏细胞, 产生并传导慢波, 还参与神经信号的传递, 控制胃肠平滑肌运动[6,16]. 研究证实ICC网络病变可导致胃肠起搏功能紊乱及电兴奋传导障碍, 胃肠平滑肌发生多种电节律紊乱(如慢波不规则或消失、收缩减弱或不能产生有效的推进性收缩), 临床上出现多种胃肠动力障碍症状; ICC的特异性标志是表达酪氨酸激酶受体c-Kit(CD117), 其胞外部分为SCF受体区, 胞内部分为酪氨酸激酶区, 很多研究已证明, 胃肠道ICC数量及结构的异常与多种胃肠疾病有关, 包括DM胃肠动力障碍、假性肠梗阻、先天性巨结肠、慢传输型便秘、食管失弛缓等[17-21]. 目前, 引起DM相关的ICC异常改变的病理生理学机制尚不清楚, 认为可能与胰岛素信号减弱、SCF减少或高糖等因素有关. 本实验结果发现DM 6 wk的小鼠远端结肠中ICC数量明显减少、ICC超微结构严重破坏, 与文献报道一致[9].
SCF是一种重要的多功能生长因子, 是c-Kit受体的天然配体, SCF与Kit结合组成Kit-SCF信号系统, 活化了酪氨酸激酶, 导致一系列磷酸化过程. Kit-SCF信号系统参与了机体所有Kit阳性细胞的发育、分化、增殖等过程[22-23], 同样也与ICC的增殖、分化和表型维持密切相关[11,24]. 小鼠SCF由10号染色体的steel(Sl)位点编码, 该编码序列缺失的成年(出生后20 d)Sl/Sld小鼠SCF合成严重受损, 胃肠道ICC难以识别[25]. Ward通过抑制3-磷脂酰醇激酶(PI3-K), 观察对新生及成年(出生后30 d)BALB/c小鼠空肠ICC的影响, 发现抑制PI3-K途径后新生小鼠和成年小鼠空肠ICC均减少, 认为PI3-K途径是SCF-Kit信号途径可能的作用环节[26]. 本实验中, DM小鼠远端结肠组织中SCF表达明显下调, 同时结肠ICC数量减少、超微结构被破坏; 而DM小鼠予以外源性SCF干预后, 结肠组织中SCF水平上调, 同时ICC数量及超微结构得到明显改善, 虽然未完全恢复到正常水平, 但该结果提示外源性SCF可以改善或逆转DM相关的结肠ICC病变; 异常的ICC未能完全恢复, 是否与干预时间、给药途径、剂量和/或信号通路等有关, 尚待验证.
SCF以两种形式存在, 即可溶型干细胞因子(soluble stem cell factor, s-SCF)(分子质量约24 kDa)和膜结合型干细胞因子(membrane-bound stem cell factor, m-SCF)(分子质量约27 kDa), 两者都有生物学活性. Rich et al[27]对BALB/c小鼠空肠ICC体外实验研究发现m-SCF对ICC有更大作用; 本实验采用的外源性SCF为重组小鼠SCF(分子质量约18 kDa), 对DM小鼠干预6 wk后, 测得结肠组织中SCF分子质量约30 kDa, 提示m-SCF表达增高, 同时使DM相关的结肠ICC病变得以改善, 提示外源性SCF可能为临床治疗DM胃肠动力障碍提供新的理论依据.
糖尿病胃肠动力障碍的发病机制目前仍不清楚, 临床上缺乏有效的治疗手段. 随着对Cajal间质细胞功能的研究日益增多, 人们对该疾病的认识不断深入, Cajal间质细胞有望成为治疗该病新的突破口.
同行评价者
关玉盘, 教授, 首都医科大学附属北京朝阳医院消化科.
近来, 干细胞因子在Cajal间质细胞以及胃肠动力障碍中的病理生理机制成为研究热点.
本研究首次在体内实验应用外源性干细胞因子对结肠Cajal间质细胞进行干预, 结果验证了干预的有效性及其对糖尿病小鼠Cajal间质细胞产生的显著影响.
本研究结果可能为临床治疗糖尿病结肠功能障碍提供新的理论依据.
c-Kit蛋白: 是已发现的Cajal间质细胞表面特异性标志物, 即酪氨酸激酶受体, 检测其含量可作为消化系Cajal间质细胞的定量指标, 干细胞因子是c-Kit的天然配基.
本课题设计及研究方法合理, 结果与结论相符, 讨论条理清晰, 具有一定的科学性和可靠性, 也具有科研和临床实用价值.
编辑: 程剑侠 电编:郭海丽
| 1. | Feldman M, Schiller LR. Disorders of gastrointestinal motility associated with diabetes mellitus. Ann Intern Med. 1983;98:378-384. [PubMed] |
| 2. | Icks A, Haastert B, Rathmann W, Wareham N. Prevalence of gastrointestinal symptoms in patients with type 2 diabetes: a population-based study. Arch Intern Med. 2002;162:1067-1069; author reply 1069. [PubMed] [DOI] |
| 3. | Maleki D, Locke GR 3rd, Camilleri M, Zinsmeister AR, Yawn BP, Leibson C, Melton LJ 3rd. Gastrointestinal tract symptoms among persons with diabetes mellitus in the community. Arch Intern Med. 2000;160:2808-2816. [PubMed] [DOI] |
| 4. | Ko GT, Chan WB, Chan JC, Tsang LW, Cockram CS. Gastrointestinal symptoms in Chinese patients with Type 2 diabetes mellitus. Diabet Med. 1999;16:670-674. [PubMed] [DOI] |
| 5. | Kito Y, Ward SM, Sanders KM. Pacemaker potentials generated by interstitial cells of Cajal in the murine intestine. Am J Physiol Cell Physiol. 2005;288:C710-C720. [PubMed] [DOI] |
| 6. | Wang XY, Sanders KM, Ward SM. Intimate relationship between interstitial cells of cajal and enteric nerves in the guinea-pig small intestine. Cell Tissue Res. 1999;295:247-256. [PubMed] [DOI] |
| 7. | Nakahara M, Isozaki K, Hirota S, Vanderwinden JM, Takakura R, Kinoshita K, Miyagawa J, Chen H, Miyazaki Y, Kiyohara T. Deficiency of KIT-positive cells in the colon of patients with diabetes mellitus. J Gastroenterol Hepatol. 2002;17:666-670. [PubMed] [DOI] |
| 8. | Forster J, Damjanov I, Lin Z, Sarosiek I, Wetzel P, McCallum RW. Absence of the interstitial cells of Cajal in patients with gastroparesis and correlation with clinical findings. J Gastrointest Surg. 2005;9:102-108. [PubMed] [DOI] |
| 9. | Long QL, Fang DC, Shi HT, Luo YH. Gastro-electric dysrhythm and lack of gastric interstitial cells of cajal. World J Gastroenterol. 2004;10:1227-1230. [PubMed] |
| 10. | Wu JJ, Rothman TP, Gershon MD. Development of the interstitial cell of Cajal: origin, kit dependence and neuronal and nonneuronal sources of kit ligand. J Neurosci Res. 2000;59:384-401. [PubMed] [DOI] |
| 11. | Sanders KM, Ward SM. Interstitial cells of Cajal: a new perspective on smooth muscle function. J Physiol. 2006;576:721-726. [PubMed] [DOI] |
| 12. | Torihashi S, Nishi K, Tokutomi Y, Nishi T, Ward S, Sanders KM. Blockade of kit signaling induces transdifferentiation of interstitial cells of cajal to a smooth muscle phenotype. Gastroenterology. 1999;117:140-148. [PubMed] [DOI] |
| 13. | Kitayama J, Faraci FM, Gunnett CA, Heistad DD. Impairment of dilator responses of cerebral arterioles during diabetes mellitus: role of inducible NO synthase. Stroke. 2006;37:2129-2133. [PubMed] [DOI] |
| 14. | Yu X, Tesiram YA, Towner RA, Abbott A, Patterson E, Huang S, Garrett MW, Chandrasekaran S, Matsuzaki S, Szweda LI. Early myocardial dysfunction in streptozotocin-induced diabetic mice: a study using in vivo magnetic resonance imaging (MRI). Cardiovasc Diabetol. 2007;6:6. [PubMed] [DOI] |
| 15. | Jung HK, Kim DY, Moon IH, Hong YS. Colonic transit time in diabetic patients--comparison with healthy subjects and the effect of autonomic neuropathy. Yonsei Med J. 2003;44:265-272. [PubMed] |
| 16. | Ito-Dufros Y, Funakoshi Y, Uehara A, Oishi K. In vitro development of gut-like tissue demonstrating rhythmic contractions from embryonic mouse intestinal cells. Neurogastroenterol Motil. 2007;19:288-300. [PubMed] [DOI] |
| 17. | He CL, Soffer EE, Ferris CD, Walsh RM, Szurszewski JH, Farrugia G. Loss of interstitial cells of cajal and inhibitory innervation in insulin-dependent diabetes. Gastroenterology. 2001;121:427-434. [PubMed] [DOI] |
| 18. | Streutker CJ, Huizinga JD, Campbell F, Ho J, Riddell RH. Loss of CD117 (c-kit)- and CD34-positive ICC and associated CD34-positive fibroblasts defines a subpopulation of chronic intestinal pseudo-obstruction. Am J Surg Pathol. 2003;27:228-235. [PubMed] [DOI] |
| 19. | Taguchi T, Suita S, Masumoto K, Nagasaki A. An abnormal distribution of C-kit positive cells in the normoganglionic segment can predict a poor clinical outcome in patients with Hirschsprung's disease. Eur J Pediatr Surg. 2005;15:153-158. [PubMed] [DOI] |
| 20. | Wedel T, Bottner M, Krammer HJ. The enteric nervous system and interstitial cells of Cajal. Changes in chronic constipation in adults. Pathologe. 2007;28:143-148. [PubMed] |
| 21. | Watanabe Y, Ando H, Seo T, Katsuno S, Marui Y, Ono Y, Torihashi S. Attenuated nitrergic inhibitory neurotransmission to interstitial cells of Cajal in the lower esophageal sphincter with esophageal achalasia in children. Pediatr Int. 2002;44:145-148. [PubMed] [DOI] |
| 22. | Li J, Goodyer CG, Fellows F, Wang R. Stem cell factor/c-Kit interactions regulate human islet-epithelial cluster proliferation and differentiation. Int J Biochem Cell Biol. 2006;38:961-972. [PubMed] [DOI] |
| 23. | Bashamboo A, Taylor AH, Samuel K, Panthier JJ, Whetton AD, Forrester LM. The survival of differentiating embryonic stem cells is dependent on the SCF-KIT pathway. J Cell Sci. 2006;119:3039-3046. [PubMed] [DOI] |
| 24. | Horvath VJ, Vittal H, Lorincz A, Chen H, Almeida-Porada G, Redelman D, Ordog T. Reduced stem cell factor links smooth myopathy and loss of interstitial cells of cajal in murine diabetic gastroparesis. Gastroenterology. 2006;130:759-770. [PubMed] [DOI] |
| 25. | Ward SM, Burns AJ, Torihashi S, Harney SC, Sanders KM. Impaired development of interstitial cells and intestinal electrical rhythmicity in steel mutants. Am J Physiol. 1995;269:C1577-C1585. [PubMed] |
| 26. | Ward SM, Brennan MF, Jackson VM, Sanders KM. Role of PI3-kinase in the development of interstitial cells and pacemaking in murine gastrointestinal smooth muscle. J Physiol. 1999;516:835-846. [PubMed] [DOI] |
| 27. | Rich A, Miller SM, Gibbons SJ, Malysz J, Szurszewski JH, Farrugia G. Local presentation of Steel factor increases expression of c-kit immuno-reactive interstitial cells of Cajal in culture. Am J Physiol Gastrointest Liver Physiol. 2003;284:G313-G320. [PubMed] |