修回日期: 2006-11-01
接受日期: 2006-11-28
在线出版日期: 2007-02-28
目的: 探索肝细胞生长因子(HGF)、制瘤素M(OSM)在体外诱导骨髓间充质干细胞(MSCs)向肝细胞分化的能力及效果.
方法: 分离、培养大鼠MSCs, 取第3代按以下分组诱导其向肝细胞分化: A组: 低糖杜氏改良培养基(DMEM-LG)+100 mL/L胎牛血清(fetal calf, serum, FCS); B组: 肝细胞生长培养基(HGM); C组: HGM+20 μg/L HGF; D组: HGM+20 μg/L OSM; E组: HGM+20 μg/L HGF+20 μg/L OSM, 于不同时间点用免疫细胞化学检测甲胎蛋白(AFP)、细胞角蛋白18(CK18)表达, 高碘酸-希夫氏(PAS)染色检测糖原表达, 谷氨酰胺脱氢酶法检测上清液尿素含量.
结果: C, E组于诱导第7天出现AFP阳性表达, 以后其阳性表达率随诱导时间延长逐渐降低, 诱导第7天E组阳性表达率高于C组(2 = 6.322, P<0.05). C组、E组分别于诱导第7、14天出现CK18阳性表达, 于诱导第7天开始出现糖原阳性表达, 随诱导时间延长表达率逐渐增高, 同一时间点E组CK18(14 d: 2 = 4.811, P<0.05; 21 d: 2 = 6.902, P<0.01; 28 d: 2 = 5.771, P<0.05)及糖原(14 d: 2 = 6.902, P<0.01; 21 d: 2 = 6.818, P<0.01; 28 d: 2 = 6.818, P<0.01)阳性表达率高于C组. C, E组培养上清液中尿素浓度随诱导时间延长逐渐增高, E组增长幅度比C组高. A, B, D组各时间点均未见AFP、CK18、糖原表达及尿素浓度变化.
结论: MSCs具有向肝细胞分化的能力, HGF能够诱导MSCs分化肝样细胞, OSM与HGF联合使用, 能促进MSCs的分化, 明显提高分化率.
引文著录: 钟晓琳, 李昌平, 胡莲, 万居易. 骨髓间充质干细胞向肝细胞的诱导分化. 世界华人消化杂志 2007; 15(6): 568-573
Revised: November 1, 2006
Accepted: November 28, 2006
Published online: February 28, 2007
AIM: To explore the capability and effect of hepatocyte growth factor (HGF) and oncostatin M (OSM) in inducing the differentiation of bone marrow mesenchymal stem cells (MSCs) into hepatocytes in vitro.
METHODS: Rat MSCs were isolated and cultured. Passage 3 MSCs were divided into group A, B, C, D and E, which were induced by dulbecco's modified eagle medium-low glucose (DMEM-LG) plus 100 mL/L fetal calf serum (FCS), hepatocyte growth medium (HGM), HGM plus 20 mg/L HGF, HGM plus 20 mg/L OSM, and HGM plus 20 mg/L HGF plus 20 mg/L OSM, respectively. The expression of alpha-fetoprotein (AFP) and cytokeratin 18 (CK18) were detected by immunocytochemistry; the glycogen deposit was examined by Periodic acid-Schiff (PAS) staining; and the urea content in culture supernatant was determined by glutamate dehydrogenases at different time points.
RESULTS: On the 7th day, the positive expression of AFP emerged in group C and E, and it was lower in the former than that in the latter (χ2 = 6.322, P < 0.05). With the extension of induction time, the expression of AFP was decreased gradually. The expression of CK18 was found in group E and C on the 7th and 14th day, respectively, and the glycogen deposit was observed on the 7th day both in group E and C. With the extension of induction time, the levels of CK18 and glycogen expression were elevated gradually. At the same time point, the expression rates of CK18 (14 d: χ2 = 4.811, P < 0.05; 21 d: χ2 = 6.902, P < 0.01; 28 d: χ2 = 5.771, P < 0.05) and glycogen (14 d: χ2 = 6.902, P < 0.01; 21 d: χ2 = 6.818, P < 0.01; 28 d: χ2 = 6.818, P < 0.01) were significantly higher in group E than those in group C. The urea concentration increased gradually with the induction time both in group C and E, but it increased faster in the latter. The expression of AFP, CK18, glucogen and the concentration of urea had no significant changes in group A, B, D.
CONCLUSION: HGF can induce the hepatocytic differentiation of MSCs, and its combination with OSM presents a better effect.
- Citation: Zhong XL, Li CP, Hu L, Wan JY. Hepatocytic differentiation of bone marrow-derived mesenchymal stem cells. Shijie Huaren Xiaohua Zazhi 2007; 15(6): 568-573
- URL: https://www.wjgnet.com/1009-3079/full/v15/i6/568.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v15.i6.568
骨髓间充质干细胞(mesenchymal stem cells, MSCs)是一种具有跨胚层多向分化潜能的成体干细胞, 在一定条件下可分化为骨、软骨、脂肪、神经元和造血基质细胞等各种体细胞[1-4]. 近年来, MSCs向肝细胞分化备受关注[5-9], 我们采用密度梯度离心法联合贴壁培养法分离、纯化MSCs, 分别使用肝细胞生长因子(hepatocyte growth factor, HGF)、制瘤素M(oncostatin M, OSM)及二者联合诱导MSCs向肝细胞分化, 并通过多种方法评价两种细胞因子的促分化能力.
8-10 wk清洁级♀Wistar大鼠, 由泸州医学院动物实验中心提供. HGF, OSM为美国R&D公司产品, 胎牛血清为杭州四季青公司产品, 低糖杜氏改良培养基(DMEM-LG)、胰岛素-转铁蛋白-亚硒酸钠(insulin-transferrin-selenium, ITS)为美国Gibco公司产品, 淋巴细胞分离液为上海试剂二厂产品, 纤维蛋白连接素(fibronectin, FN)为瑞士Roche公司产品, 小鼠抗大鼠CK18 mAb、山羊抗小鼠SABC试剂盒、DAB试剂盒购自武汉博士德生物工程公司, 山羊抗人AFP多克隆抗体购自Santa Cruz公司, 兔抗山羊SP试剂盒购自北京中山生物技术有限公司, 尿素液体试剂盒购自上海申能-德塞诊断技术有限公司.
1.2.1 MSCs的分离、培养与鉴定: 在无菌条件下取8-10 wk Wistar大鼠股骨和胫骨骨髓细胞, 采用密度梯度离心联合贴壁培养法分离、纯化MSCs, 利用MSCs与其他贴壁细胞的贴壁差异性, 严格控制传代时酶的量和消化时间, 传代纯化MSCs, 显微镜下观察细胞形态, 取第3代(passage 3, P3) MSCs, 进行成骨、成脂诱导以鉴定MSCs的多向分化能力.
1.2.2 MSCs向肝细胞的诱导分化: 肝细胞生长培养基(HGM)成分: DMEM-LG、100 mL/L FCS、地塞米松(dexamethasone, Dex)10-7 mmol/L、羟乙基哌嗪乙磺酸(hydroxyethyl piperazine ethane-sulfonic acid, HEPES)20 mmol/L、葡萄糖1 g/L、半乳糖2 g/L、ITS 10 mL/L、Vit C1 mmol/L、谷氨酰胺0.73 g/L、青霉素100 kU/L、链霉素 100 kU/L. 实验分组: A组: DMEM-LG+100 mL/L FCS; B组: HGM; C组: HGM+20 μg/L HGF; D组: HGM+20 μg/L OSM; E组: HGM+20 μg/L HGF+20 μg/L OSM. 诱导方法: 取P3 MSCs, 用2.5 g/L胰蛋白酶消化后, 以3×107/L接种于预先用50 mg/L FN处理的24孔板中, 按上述分组加入培养基及诱导因子, 于37℃、饱和湿度、50 mL/L CO2培养箱中进行诱导分化培养, 每4 d全量换液一次, 诱导细胞分化20-28 d.
1.2.3 诱导后MSCs的鉴定: 肝细胞特异性标志物检测: 采用免疫细胞化学方法于诱导第0, 7, 14, 21, 28天取细胞爬片检测其AFP、CK18表达情况; 肝细胞糖原合成功能检测: 采用高碘酸-希夫氏(PAS)染色于诱导第0, 7, 14, 21, 28天取细胞爬片, 检测诱导分化细胞糖原储存功能; 肝细胞代谢功能检测: 诱导后每4 d收集各组培养细胞上清液, 谷氨酸脱氢酶法检测上清液中尿素含量, 观察其动态变化趋势, 绘制尿素含量变化曲线.
统计学处理 实验结果计量资料以mean±SD表示, 不同时间点、不同组之间的比较采用重复测量资料的方差分析, 计数资料采用秩和检验, 以α = 0.05为检验水准, 利用SPSS13.0软件进行统计分析.
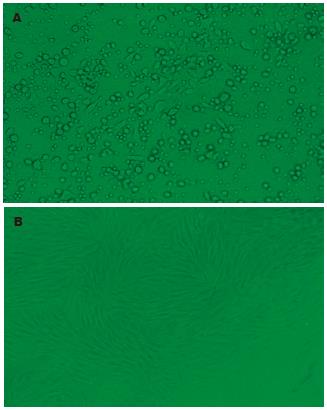
接种4-6 h后可见部分细胞贴壁, 24 h后可见呈梭形、三角形、多角形、不规则形的贴壁细胞, 5-6 d即可形成分散的成纤维细胞集落 (colony-forming units-fibroblastic, CFU-F), 10-14 d相邻集落融合成片达80%左右. 原代细胞形态多样, 分布不均, 含杂细胞多, 随换液和传代次数增多, 细胞纯度提高, P3后细胞形态一致, 呈梭形外观的贴壁细胞, 核居中, 有1-2个核仁, 几乎不见杂细胞, 分布均一, 呈漩涡状、平行状或鱼群状生长细胞排列. 经成骨诱导后, 碱性磷酸酶染色阳性, 成脂诱导后, 油红O染色阳性(图1A-B).
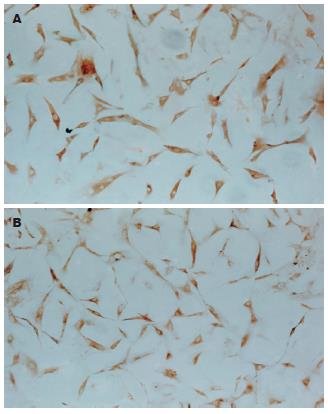
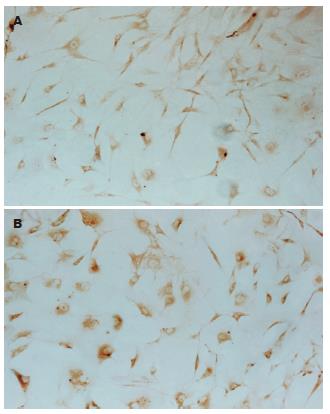
(1)AFP免疫细胞化学检测结果: AFP在诱导第0, 28天各组细胞均为阴性表达, 诱导第7, 14, 21天时C组及E组出现阳性表达, 且表达率逐渐降低(P<0.05). 诱导分化第7天, E组AFP表达率高于C组, 差别有统计学意义(P<0.05), 诱导分化第21天, E组AFP表达率低于C组, 差别有统计学意义(P<0.05). A, B, D组在7-28 d均未见阳性表达. 不加一抗对照为阴性结果(表1, 图2A-B); (2)CK18免疫细胞化学检测结果: CK18在诱导第0天各组细胞均为阴性表达, 诱导第7天E组出现CK18表达, 诱导第14, 21, 28 天HGF及E组均出现阳性表达, 随诱导时间延长, 表达率逐渐增高, 差别有统计学意义(P<0.05). 在同一时间点, E组表达率均高于C组, 差别有统计学意义(P<0.05). A, B, D组在7-28 d均未见阳性表达. 不加一抗对照为阴性结果(表2, 图3A-B).
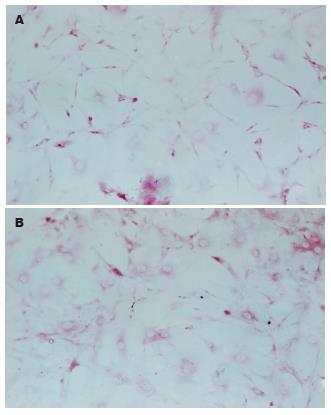
糖原在诱导第0 天各组细胞均为阴性表达, 诱导第7, 14, 21, 28 天C组及E组均出现阳性表达, 随诱导时间延长, 表达率逐渐增高, 差别有统计学意义(P<0.05). 在同一时间点, E组表达率均高于C组, 差别有统计学意义(P<0.05). A, B, D组在7-28 d均未见阳性表达. P3 MSCs对照为阴性结果(表3, 图4 A-B).
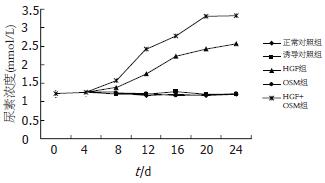
将各组各时间点上清液中尿素浓度绘制成折线图, 经统计学分析, 各组间比较差别有统计学意义(P<0.05). A, B, D组培养上清液中尿素浓度无明显变化, 各组之间无明显差异(P>0.05). 而C组及E组尿素浓度随诱导时间延长逐渐增高, 分别与A, B, D组比较, 差别有统计学意义(P<0.05), E组尿素浓度增长幅度比C组高, 差别有统计学意义(P<0.05) (图5).
MSCs在体内含量很少, 骨髓中每10万单核细胞中大约有1个MSCs[10]. 由于MSCs目前尚无特异性的表面抗原[11], 故缺乏特异的分离鉴定方法. 现有的分离方法主要有: 免疫筛选法、贴壁培养筛选法、密度梯度离心法. 鉴定方法包括: 形态学观察、联合检测多个表面标志物、多向分化的潜能鉴定等. 我们采用密度梯度离心法联合贴壁培养法简单、有效的分离、纯化MSCs, 并证实其符合MSCs的形态特点, 能分化为成骨细胞和成脂细胞, 说明我们分离培养的细胞是未分化的MSCs, 具有多向分化能力, 可以用于向肝细胞的诱导分化.
细胞因子和生长因子在组织和细胞分化中具有重要作用[12-14], 他们在骨髓干细胞向肝细胞分化中的作用的研究是目前的一个热点和难点. HGF是肝脏发育及肝再生的过程最基本的细胞因子, 他不仅能促进细胞分裂、迁移, 抑制细胞凋亡促进肝细胞增殖, 还能促进干细胞的分化[15]. HGF的受体c-met是一种膜外部分拥有酪氨酸激酶域的跨膜蛋白, 与HGF结合后, 通过受体酪氨酸激酶信号途径, 影响细胞核内的转录机构, 通过调控某些特定基因的转录和表达影响细胞增殖、分化[16-17]. 近年来的研究表明, HGF是诱导MSCs向肝细胞分化的可能的有效诱导因子之一[18-24]. OSM是IL-6家族成员之一, 他能诱导胎肝细胞分化为具有各种代谢功能的成熟肝细胞, 在体外诱导肝干细胞分化成为能产生各种代谢酶、合成白蛋白、积累糖原的成熟肝细胞, 在肝脏成熟过程中发挥重要作用[25-26]. OSM与其受体OSMR结合后, 通过非受体酪氨酸激酶信号途径发挥其生物学作用[27-29]. Lee et al[20]和Shi et al[21]的研究提示, OSM与其他细胞因子联合可以诱导骨髓干细胞分化, 但是OSM单独使用能否诱导MSCs向肝细胞的分化, 与其他细胞因子联合使用能否提高分化效率, 以及他在分化中的作用目前尚不清楚.
我们选用这两种细胞因子单独和联合使用诱导MSCs向肝细胞的分化, 通过免疫细胞化学检测不成熟肝细胞表型AFP及成熟肝细胞表型CK18的表达, 糖原染色检测诱导后细胞糖原合成能力, 生化检测培养基上清液中尿素含量的动态变化, 从细胞表型、物质合成和代谢功能等方面证实诱导后细胞是否为肝细胞. 结果显示, 单独使用HGF能够诱导MSCs向肝细胞的分化, 单独使用OSM不能诱导MSCs向肝细胞的分化, 二者联合使用能提高MSCs的分化率, 促进分化细胞的成熟. 可见, MSCs具有向肝细胞分化的能力, 单独使用OSM虽然没有诱导MSCs分化的作用, 但是与HGF联合使用能提高MSCs向肝细胞分化的效率、促进肝细胞的成熟. 同时提示我们HGF和OSM的胞内信号转导途径虽然不同[30], 但联合用于MSCs时, 其信号转导可能出现了信号交联(cross-talk), 从而影响了分化细胞的某些生物学特性, 增强了HGF促进MSCs向肝细胞分化的生物学效应. 为我们下一步研究提供了新的思路.
总之, MSCs具有自体取材、体外分离培养容易, 增殖、分化能力强等优点, 是最具有治疗潜能的种子细胞. 使用HGF与OSM联合诱导MSCs向肝细胞的分化是可行的, 可能为肝细胞移植及生物人工肝提供种子细胞, 以期治疗各种终末期肝病.
肝细胞移植(HCT) 是治疗终末期肝功能衰竭的最有效手段之一, 但却受到细胞来源缺乏、免疫排斥反应等多种因素限制而影响其临床应用. 骨髓间充质干细胞(MSCs)具有多向分化能力, 可能为肝细胞移植提供丰富的自体肝细胞来源.
MSC s具有多向分化潜能, 其分化方向由其所处的微环境决定, 且 MSCs在骨髓中含量很少, 因此, 建立MSCs有效的体外分离、扩增方法, 研究MSCs定向分化的影响因素是实现MSCs向肝细胞分化的前提和关键. 细胞因子和生长因子在组织和细胞分化中具有重要作用, 他们在骨髓干细胞向肝细胞分化中的作用的研究是目前的一个热点和难点.
近来的研究表明MSC s在一定条件下能分化为肝细胞, HGF, OSM, FGF-4, EGF等多种细胞因子都可能在诱导其向肝细胞分化中起关键作用, 但是各种因素具体的作用及何种因素才是诱导MSCs分化的理想因素目前还不清楚, 因此需进一步明确相关细胞因子在MSCs分化中的作用.
HGF是肝脏发育及肝再生的过程最基本的细胞因子, 也是诱导 MSCs向肝细胞分化的可能的有效诱导因子, OSM在肝脏成熟发育过程中起重要作用, 与其他细胞因子联合可以诱导MSCs分化, 但 HGF、OSM单独使用诱导MSCs向肝细胞的分化的能力, 以及联合 HGF使用能否提高分化效率、促进肝细胞成熟, 国内外尚无报道.
本文探讨MSCs向肝细胞分化的能力及HGF, OSM诱导分化中的作用, 立题思路清晰, 设计科学, 研究方法可靠, 结果有创新性和参考意义.
电编: 李琪 编辑:王晓瑜
| 1. | Rojas M, Xu J, Woods CR, Mora AL, Spears W, Roman J, Brigham KL. Bone marrow-derived mesenchymal stem cells in repair of the injured lung. Am J Respir Cell Mol Biol. 2005;33:145-152. [PubMed] |
| 2. | Moreau JE, Chen J, Bramono DS, Volloch V, Chernoff H, Vunjak-Novakovic G, Richmond JC, Kaplan DL, Altman GH. Growth factor induced fibroblast differentiation from human bone marrow stromal cells in vitro. J Orthop Res. 2005;23:164-174. [PubMed] |
| 3. | Wang Y, Deng Z, Lai X, Tu W. Differentiation of human bone marrow stromal cells into neural-like cells induced by sodium ferulate in vitro. Cell Mol Immunol. 2005;2:225-229. [PubMed] |
| 4. | Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143-147. [PubMed] |
| 5. | Seo MJ, Suh SY, Bae YC, Jung JS. Differentiation of human adipose stromal cells into hepatic lineage in vitro and in vivo. Biochem Biophys Res Commun. 2005;328:258-264. [PubMed] |
| 6. | Hong SH, Gang EJ, Jeong JA, Ahn C, Hwang SH, Yang IH, Park HK, Han H, Kim H. In vitro differentiation of human umbilical cord blood-derived mesenchymal stem cells into hepatocyte-like cells. Biochem Biophys Res Commun. 2005;330:1153-1161. [PubMed] |
| 7. | Wang PP, Wang JH, Yan ZP, Hu MY, Lau GK, Fan ST, Luk JM. Expression of hepatocyte-like phenotypes in bone marrow stromal cells after HGF induction. Biochem Biophys Res Commun. 2004;320:712-716. [PubMed] |
| 8. | Zhang Y, Fan Y, Zhao L, Tang H. Differentiation of mouse bone marrow mesenchymal stem cells into hepatocyte in vivo. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2005;22:521-524. [PubMed] |
| 10. | Ohgushi H, Caplan AI. Stem cell technology and bioceramics: from cell to gene engineering. J Biomed Mater Res. 1999;48:913-927. [PubMed] |
| 11. | Majumdar MK, Thiede MA, Mosca JD, Moorman M, Gerson SL. Phenotypic and functional comparison of cultures of marrow-derived mesenchymal stem cells (MSCs) and stromal cells. J Cell Physiol. 1998;176:57-66. [PubMed] |
| 12. | Schwartz RE, Reyes M, Koodie L, Jiang Y, Blackstad M, Lund T, Lenvik T, Johnson S, Hu WS, Verfaillie CM. Multipotent adult progenitor cells from bone marrow differentiate into functional hepatocyte-like cells. J Clin Invest. 2002;109:1291-1302. [PubMed] |
| 13. | Tang LJ, Gao Y, Zhang Z, Li H, Shan YQ. Human bone marrow multipotent adult progenitor cells differentiate into hepatocyte-like cells with hepatocyte growth factor plus fibroblast growth factor-4 in vitro. Zhonghua Gan Zang Bing Za Zhi. 2005;13:652-655. [PubMed] |
| 14. | Lange C, Bassler P, Lioznov MV, Bruns H, Kluth D, Zander AR, Fiegel HC. Hepatocytic gene expression in cultured rat mesenchymal stem cells. Transplant Proc. 2005;37:276-279. [PubMed] |
| 15. | Defrances MC, Wolf HK, Michalopoulos GK, Zarnegar R. The presence of hepatocyte growth factor in the developing rat. Development. 1992;116:387-395. [PubMed] |
| 16. | Cartwright JE, Tse WK, Whitley GS. Hepatocyte growth factor induced human trophoblast motility involves phosphatidylinositol-3-kinase, mitogen-activated protein kinase, and inducible nitric oxide synthase. Exp Cell Res. 2002;279:219-226. [PubMed] |
| 17. | Duan HF, Wu CT, Lu Y, Wang H, Liu HJ, Zhang QW, Jia XX, Lu ZZ, Wang LS. Sphingosine kinase activation regulates hepatocyte growth factor induced migration of endothelial cells. Exp Cell Res. 2004;298:593-601. [PubMed] |
| 18. | Oh SH, Miyazaki M, Kouchi H, Inoue Y, Sakaguchi M, Tsuji T, Shima N, Higashio K, Namba M. Hepatocyte growth factor induces differentiation of adult rat bone marrow cells into a hepatocyte lineage in vitro. Biochem Biophys Res Commun. 2000;279:500-504. [PubMed] |
| 19. | Fiegel HC, Lioznov MV, Cortes-Dericks L, Lange C, Kluth D, Fehse B, Zander AR. Liver-specific gene expression in cultured human hematopoietic stem cells. Stem Cells. 2003;21:98-104. [PubMed] |
| 20. | Lee KD, Kuo TK, Whang-Peng J, Chung YF, Lin CT, Chou SH, Chen JR, Chen YP, Lee OK. In vitro hepatic differentiation of human mesenchymal stem cells. Hepatology. 2004;40:1275-1284. [PubMed] |
| 21. | Shi XL, Qiu YD, Li Q, Xie T, Zhu ZH, Chen LL, Li L, Ding YT. Hepatocyte-like cells from directed differentiation of mouse bone marrow cells in vitro. Acta Pharmacol Sin. 2005;26:469-476. [PubMed] |
| 22. | Miyazaki M, Akiyama I, Sakaguchi M, Nakashima E, Okada M, Kataoka K, Huh NH. Improved conditions to induce hepatocytes from rat bone marrow cells in culture. Biochem Biophys Res Commun. 2002;298:24-30. [PubMed] |
| 24. | Oyagi S, Hirose M, Kojima M, Okuyama M, Kawase M, Nakamura T, Ohgushi H, Yagi K. Therapeutic effect of transplanting HGF-treated bone marrow mesenchymal cells into CCl4-injured rats. J Hepatol. 2006;44:742-748. [PubMed] |
| 25. | Kamiya A, Kinoshita T, Ito Y, Matsui T, Morikawa Y, Senba E, Nakashima K, Taga T, Yoshida K, Kishimoto T. Fetal liver development requires a paracrine action of oncostatin M through the gp130 signal transducer. EMBO J. 1999;18:2127-2136. [PubMed] |
| 26. | Kamiya A, Gonzalez FJ, Nakauchi H. Identification and differentiation of hepatic stem cells during liver development. Front Biosci. 2006;11:1302-1310. [PubMed] |
| 27. | Nightingale J, Patel S, Suzuki N, Buxton R, Takagi KI, Suzuki J, Sumi Y, Imaizumi A, Mason RM, Zhang Z. Oncostatin M, a cytokine released by activated mononuclear cells, induces epithelial cell-myofibroblast transdifferentiation via Jak/Stat pathway activation. J Am Soc Nephrol. 2004;15:21-32. [PubMed] |
| 28. | Nakamura K, Nonaka H, Saito H, Tanaka M, Miyajima A. Hepatocyte proliferation and tissue remodeling is impaired after liver injury in oncostatin M receptor knockout mice. Hepatology. 2004;39:635-644. [PubMed] |