修回日期: 2007-10-07
接受日期: 2007-10-28
在线出版日期: 2007-10-28
目的: 探讨p38MPAK是否参与Fas和AD诱导Bel-7402细胞的凋亡过程, 以及p38MPAK和bcl-2的关系, 进一步揭示p38MAPK的凋亡途径.
方法: 在Fas和AD作用24 h后, 用MTT法检测Bel-7402细胞的活力, 用Western-blot和RT-PCR法检测p38MAPK, p-p38MAPK和Bcl-2 expression, 用免疫荧光法对p-p38MAPK进行细胞定位.
结果: 随着Fas浓度的增加, Bel-7402细胞的活力明显抑制, p38MAPK和p-p38MAPK表达明显增高(P<0.01), 且p-p38MAPK由胞质易位到胞核. Bcl-2的表达明显降低(P<0.01), 并且这种降低趋势被p38MAPK抑制剂SB203580所阻止.
结论: p38MAPK参与Fas诱导的凋亡途径, 以磷酸化形式激活后抑制Bcl-2的表达, 进而促进细胞凋亡.
引文著录: 王玉, 孙黎光, 夏春辉, 叶丽平, 张莹. 在Fas诱导Bel-7402细胞凋亡中p38MAPK调节Bcl-2的表达. 世界华人消化杂志 2007; 15(30): 3184-3189
Revised: October 7, 2007
Accepted: October 28, 2007
Published online: October 28, 2007
AIM: To investigate whether p38 mitogen-activated protein kinase (p38MAPK) is involved in Fas- and actinomycin D (AD)-induced apoptosis in Bel-7402 cells, and the relationship between p38MAPK and Bcl-2 expression.
METHODS: We measured the viability of Bel-7402 cells by MTT assay, p38MAPK, p-p38MAPK and Bcl-2 expression by Western blotting and reverse transcription polymerase chain reaction (RT-PCR), and the location of p-p38MAPK in Bel-7402 cells after Fas and AD treatment by immunofluorescene.
RESULTS: Bel-7402 cell viability was significantly inhibited by Fas (P < 0.01). p38MAPK and p-p38MAPK increased significantly with increasing Fas (P < 0.01), leading to cell death as assessed by MTT after 24 hours of Fas and AD treatment. p-p38MAPK translocation into the nucleus was dependent on Fas stimulation. Bcl-2 expression decreased significantly and was prevented by SB203580 during Fas- and AD-induced apoptosis (P < 0.01).
CONCLUSION: p38MAPK is involved in Fas- and AD-induced apoptosis, and p38MAPK modulates Bcl-2 expression during this apoptosis pathway.
- Citation: Wang Y, Sun LG, Xia CH, Ye LP, Zhang Y. p38 mitogen-activated protein kinase modulates Bcl-2 expression during FAS-induced apoptosis in Bel-7402 cells. Shijie Huaren Xiaohua Zazhi 2007; 15(30): 3184-3189
- URL: https://www.wjgnet.com/1009-3079/full/v15/i30/3184.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v15.i30.3184
已有研究表明, 在各种疾病如癌症、自身免疫、不规则的神经变性和生理细胞死亡中, 细胞凋亡起到重要作用, 并且许多因子都卷入死亡信号中. 近年来发现一类新的MAPK(丝裂素活化的蛋白激酶)通路-p38MAPK信号通路, 他不仅在炎症、应激反应中具有重要作用, 还参与细胞的生存、分化和凋亡等过程. p38MAPK信号通路与TNF 共同参与对细胞凋亡的调控, 是细胞内主要的信号转导系统之一. 细胞运用这一系统将胞外信号传递给胞核, 参与和影响细胞的多种生理病理过程[1-2]. Fas/FasL系统在凋亡的过程中起到关键作用. 一些证据显示, 在肝细胞凋亡的调节中Fas是非常重要的, 在肝病如肝损伤, 肝炎, 肝硬化和肝癌等的发病机制上起到重要作用[3-4]. 在正常的鼠和人的肝中, Nomura et al发现Fas不断表达, 添加的anti-Fas抗体, 可导致鼠或人的肝细胞凋亡[5]. Fas/FasL系统作为肿瘤治疗的一个潜在靶子[6-7], 不仅对组织细胞的限制或肿瘤细胞的增殖, 而且对免疫反应的下游调节都是一个生理性关键[8-9]. 我们主要探讨Fas诱导Bel-7402细胞凋亡中p38MAPK的作用途径.
MTT和胰酶均购自美国Sigma公司, DMEM培养基购自美国Gibco公司, 胎牛血清购自PAA. Fas, p38, p-p38, Bcl-2和β-actin抗体和TITC标记的二抗购自美国Santa Cruz公司, p38MAPK抑制剂SB 203580和Actinomycin D(AD)购自CalBiochem. TRIzol购自Invitrogen公司. Bel-7402细胞购自中科院动物研究所.
1.2.1 Fas和AD对细胞增殖的抑制作用: 取对数生长期的Bel-7402细胞, 常规胰酶消化接种于96孔板, 每孔1×104个细胞, 培养24 h后加入Fas和AD, Fas终浓度为0, 100, 300, 500 µg/L, AD终浓度为50 µg/L, 每个浓度设3个平行孔. 培养24 h后加入5 g/L MTT 20 µL, 再培养4 h后, 各孔加150 µL DMSO, 酶标仪检测, 波长490 nm. 细胞活力 = (实验组A值/对照组A值)×100%[10-11].
1.2.2 p38, p-p38MAPK, Bcl-2的表达: Fas和AD作用Bel-7402细胞24 h后, 收集细胞提取总蛋白, 用lorry法测定含量后, 取总蛋白30 µg, 经120 g/L SDS-聚丙烯酰胺凝胶电泳分离并转移至硝酸纤维素膜上. 用含50 g/L奶粉的TBST室温封闭1 h, 分别加1:400稀释的兔抗人p38MAPK, p-p38MAPK与Bcl-2抗体, 室温孵育2 h; 用TBST漂洗10 min, 3次; 加1:5000稀释的过氧化物酶标记的二抗, 室温孵育1 h; 用TBST漂洗10 min, 2次, TBS漂洗1次, ECL发光[12].
1.2.3 p-p38的定位: 常规培养Bel-7402细胞, 按1×105个/孔接种于放有盖玻片的6孔细胞培养板. 24 h后用含不同剂量Fas: 0, 100, 300, 500 µg/L, AD: 50 µg/L和DMEM处理24 h. 盖玻片丙酮固定, 然后抗p-p38MAPK的一抗室温孵育1 h, TRITC标记的二抗于常温避光孵育1 h后, PBS洗2次, 置Leica SP2激光共聚焦扫描显微镜下观察.
1.2.4 RT-PCR[12]采用TaKaRa试剂盒(#AMV 3.0): PCRs反应条件: 35 cycles, 变性30 s 94℃, 退火30 s 55℃, 延伸1 min 72℃, 另加延伸10 min 72℃. p38MAPK引物序列(5'-3')F-GCT CAG TAA CAG TCC GCC TAG A-, R-GAG CCA GTC CAA AAT CCA-, β-actin引物序列(5'-3')F-TCC TCC TGA GCG CAA GTA CTC T-, R- GCT CAG TAA CAG TCC GCC TAG A-. PCR产物进行15 g/L琼脂糖电泳, 用上海天能凝胶成像图像分析系统采集图像.
统计学处理 采用SPSS12.0统计软件进行统计分析.
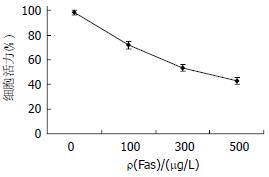
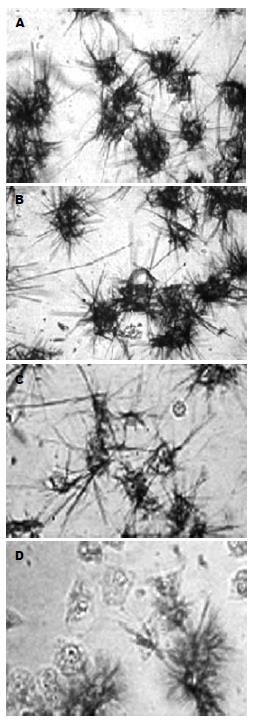
随着Fas抗体浓度的增加, 细胞的活力明显降低(97.5%→42.3%, P<0.01), 当浓度达到500 µg/L时, 这种降低趋势缓解(P<0.05), 说明在AD存在的情况下, Fas诱导细胞凋亡(图1). 另外在倒置显微镜下, 根据生成Formazan紫蓝色结晶(松针状结构)的多少进行细胞活力的判断, 我们也能很好的观察到这种效果(图2).
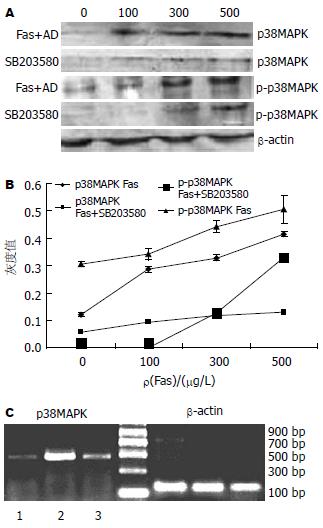
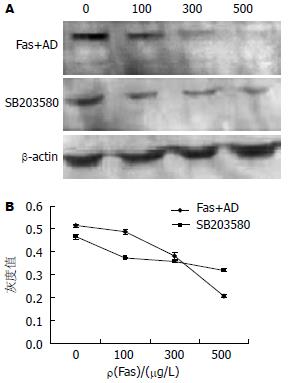
Fas和AD促进p38MAPK表达, 并且通过磷酸化(Tyr-182)形式激活, 促进p-p38MAPK表达, 随着Fas浓度的增加. p38MAPK的表达显著增加(0.126→0.417, P<0.01), p-p38MAPK的表达也显著增加(0.304→0.592, P<0.01, 图3A-B). 而抑制剂SB203580能有效的抑制p38MAPK(P<0.01)和p-p38MAPK的表达(P<0.01, 图3A-B). SB203580可以从转录水平影响p38MAPK mRNA的表达, 从而在蛋白水平表现变化(图3C).
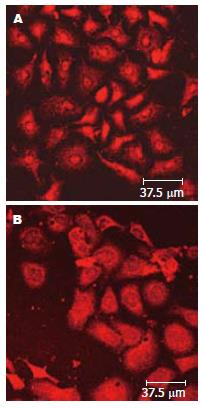
Fas和AD作用24 h后, p-p38MAPK在核内表达(TRITC标记的红色, 图4A). 当SB203580提前作用2 h后, 再用Fas和AD作用24 h, p-p38MAPK在胞质表达较多(TRITC标记的红色, 图4B).
细胞凋亡已成为一种维持机体自身稳定所必须的生理性细胞死亡. 机体内细胞在凋亡过程中, 严格受到基因调控, 因此有学者提出, 细胞恶性转化或癌细胞的产生均是由细胞增殖与细胞死亡调控功能失调所致. 参与调节细胞凋亡的许多信号途径中, Fas/FasL是最重要的介导细胞凋亡系统[13]. Fas/FasL的研究意义, 一个主要的方面就是与抗肿瘤治疗有关[30]. 已有报道, p38MAPK参与了此凋亡途径. MAPK 的激活是通过Linker12磷酸化环状结构上的三肽基"T2X2Y"中邻近的酪氨酸(T)和苏氨酸(Y)的磷酸化来完成. p38MAPK具有这样的"T2G2Y"三肽序列, 且在Linker 12部位比RasPERK, JNKPSAPK少6个氨基酸, 提示p38MAPK分子的磷酸化有别于其他3条通路[14]. p38家族有p38α, p38β, p38γ和p38δ 4种亚型, 除了序列相似之外, 同源激酶之间有一定的差异. p38α和p38β几乎表达于所有组织, 而p38γ mRNA主要在骨骼肌表达, p38δ主要在肾和肺组织内表达[15-18]. 因此对4种亚型在细胞定位的研究将有助了解p38信号通路主要参与应激条件下细胞炎症反应和细胞凋亡过程[19-21]. 根据文献报道和本研究证明, 只有在AD存在下, Fas诱导Bel-7402细胞的活力降低, 并且随Fas浓度的增加而显著降低(图1), 这种趋势在Fas浓度为500 µg/L时减缓(P<0.05), 这与细胞膜表面的Fas受体的饱和程度有关.
我们的研究也证实, p38MAPK通路的激活与外界的刺激有关. 在Fas和AD诱导的凋亡中, 随着Fas浓度的增加, p38MAPK及p-p38MAPK的表达显著增加(图3). 在正常的Bel-7402细胞中p38MAPK及p-p38MAPK的表达量很低, 在SB203580的作用下, p38MAPK及p-p38MAPK(Tyr-182)表达降低, 相应的Fas和AD诱导的凋亡也降低, 说明p38MAPK参与Fas诱导的凋亡途径. 另外从RT-PCR我们也看到, SB203580降低了p38MAPK mRNA的表达, 因而在蛋白水平得以体现与Nicholas Farley研究相一致[13]. 再有, 通过激光共聚焦扫描显微镜, 我们观察到Fas和AD作用下p-p38MAPK主要定位在胞核, 而在SB203580作用下, p-p38MAPK主要定位在胞浆. 由此, 我们推断Fas和AD刺激p38MAPK磷酸化激活, 通过p-p38MAPK将信号带入细胞核从而引起细胞凋亡.
凋亡抗在抗癌药物作用中起到非常重要的作用, 维持内环境的稳定, 消除细胞的损伤[23-24]. 两个主要的途径死亡受体途径和线粒体途径已经被证实与药物诱导的凋亡有关, 尤其, Bcl-2家族蛋白在调节线粒体凋亡途径中起到一个中心作用[25-26]. Bcl-2家族蛋白的成员可以被分为两个亚家族成员: 一个是抗凋亡蛋白如Bcl-2和Bcl-XL, 另一个是促凋亡蛋白Bax, Bad和Bid. 近来的研究表明, 在凋亡中, Bcl-2或Bcl-XL的过度表达, 能保护抵抗化学疗法诱导线粒体细胞色素C的释放, caspase的激活和DNA断裂[27]. 细胞质的促凋亡蛋白Bid能被激活的caspase-8分开和激活. 截断的Bid转位到线粒体, 然后诱导C的释放. Bcl-2 是研究最早的抑制凋亡的基因[28], 可以阻断细胞程序化死亡的公共信号传导通路[29], 因此,可以抑制或阻断多种细胞及细胞系的细胞程序化死亡过程, 但其作用机制还不清楚. 我们的数据显示, Fas抗体浓度的升高与Bcl-2原癌基因的表达下降具有某些偶联的关系. 当SB203580作用时, 我们发现Bcl-2表达下降的趋势缓解, 说明SB203580调节bcl-2的表达, 即p38MAPK参与对Bcl-2的调节.
我们的研究显示, 在AD存在下, Fas诱导Bel-7402细胞凋亡, 并且p38MAPK参与此过程. 通过p38MAPK特异性抑制剂SB203580的作用, 证明p38MAPK对Bcl-2的调节作用, 但是以依赖线粒体途径还是依非线粒体途径还不清楚, 这是我们下一个将研究的目标.
p38MAPK信号通路与Fas共同参与对细胞凋亡的调控, 是细胞内主要的信号转导系统之一. 细胞运用这一系统将胞外信号传递给胞核, 参与和影响细胞的多种生理病理过程, 但是其作用途径还不太清楚.
国外对此方面的研究采用基因敲除, 或转染方法, 具有一定的先进性, 准确性.
本文创新点在于利用p38MAPK特异性抑制剂SB203580研究p38MAPK对bcl-2的调节作用, 为今后的工作做铺垫.
p38MAPK信号通路的研究为以后肿瘤的临床治疗提供可靠的依据.
1 MAPK: 有丝分裂原蛋白激酶, MAPK包括细胞外信号调节激酶(ERK), c-Jun NH2-端蛋白激酶(JNK)和P38蛋白激酶, ERK主要受生长因子激活, 并且显示出与细胞增殖与分化相偶联. 相反致炎因子(炎症前因子), 去除生长因子, 化学治疗药物和许多有毒的化合物通常刺激JNK和P38的激活. 2 Fas: 即Apo-1/CD95, 属于肿瘤坏死因子受体(TNFR)超家族. Fas与其配体( FasL)结合后可以导致细胞凋亡, 在维持机体自身稳定和保持内环境的平衡方面起重要作用.
本文研究的内容具有一定的前沿性, 研究角度也具有创新性.
编辑: 何燕 电编:李军亮
| 1. | MacCorkle RA, Tan TH. Mitogen-activated protein kinases in cell-cycle control. Cell Biochem Biophys. 2005;43:451-461. [PubMed] |
| 2. | Bendotti C, Atzori C, Piva R, Tortarolo M, Strong MJ, DeBiasi S, Migheli A. Activated p38MAPK is a novel component of the intracellular inclusions found in human amyotrophic lateral sclerosis and mutant SOD1 transgenic mice. J Neuropathol Exp Neurol. 2004;63:113-119. [PubMed] |
| 3. | Scholz M, Cinatl J. Fas/FasL interaction: a novel immune therapy approach with immobilized biologicals. Med Res Rev. 2005;25:331-342. [PubMed] |
| 4. | Socolovsky M, Murrell M, Liu Y, Pop R, Porpiglia E, Levchenko A. Negative Autoregulation by FAS Mediates Robust Fetal Erythropoiesis. PLoS Biol. 2007;5:e252. [PubMed] |
| 5. | Nomura J, Matsumoto K, Iguchi-Ariga SM, Ariga H. Mitochondria-independent induction of Fas-mediated apoptosis by MSSP. Oncol Rep. 2005;14:1305-1309. [PubMed] |
| 6. | Scholz M, Simon A, Berg M, Schuller AM, Hacibayramoglu M, Margraf S, Theisen A, Windolf J, Wimmer-Greinecker G, Moritz A. In vivo inhibition of neutrophil activity by a FAS (CD95) stimulating module: arterial in-line application in a porcine cardiac surgery model. J Thorac Cardiovasc Surg. 2004;127:1735-1742. [PubMed] |
| 7. | Wang XB, Zhao BF, Zhao Q, Piao JH, Liu J, Lin Q, Huang HL. A new recombinant single chain trispecific antibody recruits T lymphocytes to kill CEA (carcinoma embryonic antigen) positive tumor cells in vitro efficiently. J Biochem (Tokyo). 2004;135:555-565. [PubMed] |
| 8. | Scheel-Toellner D, Wang K, Craddock R, Webb PR, McGettrick HM, Assi LK, Parkes N, Clough LE, Gulbins E, Salmon M. Reactive oxygen species limit neutrophil life span by activating death receptor signaling. Blood. 2004;104:2557-2564. [PubMed] |
| 9. | Pearl-Yafe M, Yolcu ES, Stein J, Kaplan O, Shirwan H, Yaniv I, Askenasy N. Expression of Fas and Fas-ligand in donor hematopoietic stem and progenitor cells is dissociated from the sensitivity to apoptosis. Exp Hematol. 2007;35:1601-1612. [PubMed] |
| 10. | Hou DX, Fukuda M, Johnson JA, Miyamori K, Ushikai M, Fujii M. Fisetin induces transcription of NADPH:quinone oxidoreductase gene through an antioxidant responsive element-involved activation. Int J Oncol. 2001;18:1175-1179. [PubMed] |
| 11. | Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55-63. [PubMed] |
| 12. | Hou DX, Ose T, Lin S, Harazoro K, Imamura I, Kubo M, Uto T, Terahara N, Yoshimoto M, Fujii M. Anthocyanidins induce apoptosis in human promyelocytic leukemia cells: structure-activity relationship and mechanisms involved. Int J Oncol. 2003;23:705-712. [PubMed] |
| 13. | Saitou Y, Shiraki K, Yamanaka T, Miyashita K, Inoue T, Yamanaka Y, Yamaguchi Y, Enokimura N, Yamamoto N, Itou K. Augmentation of tumor necrosis factor family-induced apoptosis by E3330 in human hepatocellular carcinoma cell lines via inhibition of NF kappa B. World J Gastroenterol. 2005;11:6258-6261. [PubMed] |
| 14. | Jin Y, Yan EZ, Fan Y, Zong ZH, Qi ZM, Li Z. Sodium ferulate prevents amyloid-beta-induced neurotoxicity through suppression of p38 MAPK and upregulation of ERK-1/2 and Akt/protein kinase B in rat hippocampus. Acta Pharmacol Sin. 2005;26:943-951. [PubMed] |
| 15. | Jiang Y, Chen C, Li Z, Guo W, Gegner JA, Lin S, Han J. Characterization of the structure and function of a new mitogen-activated protein kinase (p38beta). J Biol Chem. 1996;271:17920-17926. [PubMed] |
| 16. | Jiang Y, Gram H, Zhao M, New L, Gu J, Feng L, Di Padova F, Ulevitch RJ, Han J. Characterization of the structure and function of the fourth member of p38 group mitogen-activated protein kinases, p38delta. J Biol Chem. 1997;272:30122-30128. [PubMed] |
| 17. | Li Z, Jiang Y, Ulevitch RJ, Han J. The primary structure of p38 gamma: a new member of p38 group of MAP kinases. Biochem Biophys Res Commun. 1996;228:334-340. [PubMed] |
| 18. | Cuenda A, Rousseau S. p38 MAP-kinases pathway regulation, function and role in human diseases. Biochim Biophys Acta. 2007;1773:1358-1375. [PubMed] |
| 19. | Aouadi M, Binetruy B, Caron L, Le Marchand-Brustel Y, Bost F. Role of MAPKs in development and differentiation: lessons from knockout mice. Biochimie. 2006;88:1091-1098. [PubMed] |
| 20. | Iyoda K, Sasaki Y, Horimoto M, Toyama T, Yakushijin T, Sakakibara M, Takehara T, Fujimoto J, Hori M, Wands JR. Involvement of the p38 mitogen-activated protein kinase cascade in hepatocellular carcinoma. Cancer. 2003;97:3017-3026. [PubMed] |
| 21. | Uddin S, Ah-Kang J, Ulaszek J, Mahmud D, Wickrema A. Differentiation stage-specific activation of p38 mitogen-activated protein kinase isoforms in primary human erythroid cells. Proc Natl Acad Sci U S A. 2004;101:147-152. [PubMed] |
| 22. | Farley N, Pedraza-Alva G, Serrano-Gomez D, Nagaleekar V, Aronshtam A, Krahl T, Thornton T, Rincon M. p38 mitogen-activated protein kinase mediates the Fas-induced mitochondrial death pathway in CD8+ T cells. Mol Cell Biol. 2006;26:2118-2129. [PubMed] |
| 23. | Wesche-Soldato DE, Swan RZ, Chung CS, Ayala A. The apoptotic pathway as a therapeutic target in sepsis. Curr Drug Targets. 2007;8:493-500. [PubMed] |
| 24. | Ayala A, Wesche-Soldato DE, Perl M, Lomas-Neira JL, Swan R, Chung CS. Blockade of apoptosis as a rational therapeutic strategy for the treatment of sepsis. Novartis Found Symp. 2007;280:37-49; discussion 49-52, 160-164. [PubMed] |
| 25. | Breckenridge DG, Germain M, Mathai JP, Nguyen M, Shore GC. Regulation of apoptosis by endoplasmic reticulum pathways. Oncogene. 2003;22:8608-8618. [PubMed] |
| 26. | Daniel PT, Schulze-Osthoff K, Belka C, Guner D. Guardians of cell death: the Bcl-2 family proteins. Essays Biochem. 2003;39:73-88. [PubMed] |
| 27. | Hasenjager A, Gillissen B, Muller A, Normand G, Hemmati PG, Schuler M, Dorken B, Daniel PT. Smac induces cytochrome c release and apoptosis independently from Bax/Bcl-x(L) in a strictly caspase-3-dependent manner in human carcinoma cells. Oncogene. 2004;23:4523-4535. [PubMed] |
| 28. | Dasmahapatra G, Almenara JA, Grant S. Flavopiridol and histone deacetylase inhibitors promote mitochondrial injury and cell death in human leukemia cells that overexpress Bcl-2. Mol Pharmacol. 2006;69:288-298. [PubMed] |
| 29. | Robertson JD, Orrenius S. Molecular mechanisms of apoptosis induced by cytotoxic chemicals. Crit Rev Toxicol. 2000;30:609-627. [PubMed] |
| 30. | Pearl-Yafe M, Yolcu ES, Stein J, Kaplan O, Yaniv I, Shirwan H, Askenasy N. Fas ligand enhances hematopoietic cell engraftment through abrogation of alloimmune responses and nonimmunogenic interactions. Stem Cells. 2007;25:1448-1455. [PubMed] |