修回日期: 2007-10-08
接受日期: 2007-10-28
在线出版日期: 2007-10-28
目的: 探讨高迁移率族蛋白B1在重症急性胰腺炎(SAP)肠屏障损害中的作用及其机制.
方法: 采用逆行胆胰管注射50 g/L牛磺胆酸钠制备SAP大鼠模型. 将56只Wistar大鼠随机分为正常对照组(Control组), SAP 3 h, 6 h, 12 h, 24 h, 48 h 5个亚组, 二硫代氨基甲酸吡咯烷处理组(PDTC组), 每组8只. PDTC组于建模后24 h取材. 测定血浆内毒素(LPS)、 二胺氧化酶(DAO)水平, 用逆转录-聚合酶链反应(RT-PCR)方法检测肠组织高迁移率族蛋白B1(HMGB1) mRNA表达, 用Western blot法检测肠组织HMGB1水平.
结果: 与对照组比较, SAP 6 h组大鼠肠组织HMGB1 mRNA表达显著增高(0.41±0.06 vs 0.26±0.03, P<0.01), 12 h呈现进一步升高趋势, 24 h达峰值(0.62±0.06), 并持续至48 h. PDTC干预可显著降低肠组织HMGB1 mRNA表达(0.35±0.06 vs 0.62±0.06, P<0.01). PDTC组较SAP 24 h组肠组织HMGB1 mRNA表达, 血浆LPS和DAO显著下降(HMGB1 mRNA表达: 0.35±0.06 vs 0.62±0.06, P<0.01; LPS: 0.433±0.120 KEU/L vs 0.852±0.232 KEU/L, P<0.01; DAO: 0.65±0.12 kU/L vs 1.36±0.22 kU/L, P<0.01).
结论: SAP时, 肠组织内HMGB1表达上调; PDTC可以明显抑制SAP时肠组织内HMGB1表达.
引文著录: 栾正刚, 张成, 葛春林, 马晓春, 郭仁宣. 高迁移率族蛋白B1在重症急性胰腺炎肠屏障损害中的作用. 世界华人消化杂志 2007; 15(30): 3173-3177
Revised: October 8, 2007
Accepted: October 28, 2007
Published online: October 28, 2007
AIM: To investigate the role of high mobility group box-1 protein (HMGB1) in gut mucosal barrier dysfunction during severe acute pancreatitis (SAP) in mice, and the mechanisms involved.
METHODS: A rat model of SAP was established by retrograde injection of 50 g/L sodium taurocholate into the choledochopancreatic duct. Fifty-six healthy male Wistar rats were divided randomly into three groups: control, SAP (3, 6, 12, 24 and 48 hours subgroups), and pyrrolidine dithiocarbamate (PDTC). Plasma lipopolysaccharide (LPS) and blood diamine oxidase (DAO) levels were determined. The expression of HMGB1 mRNA in the intestinal mucosa was detected by reverse-transcription polymerase chain reaction (RT-PCR), and the activity of HMGB1 was determined by Western blotting.
RESULTS: Compared with the normal control group, HMGB1 mRNA level markedly increased in intestinal mucosa 6 hours after SAP (0.41 ± 0.06 vs 0.26 ± 0.03, P < 0.01), peaked at 24 hours (0.62 ± 0.06), and remained relatively high up to 48 hours. Meanwhile, HMGB1 mRNA expression was significantly inhibited by PDTC in the intestine 24 hours after SAP (0.35 ± 0.06 vs 0.62 ± 0.06, P < 0.01). PDTC alleviated the blood level of endotoxin and DAO 24 hours after SAP (LPS, 0.433 ± 0.120 KEU/L vs 0.852 ± 0.232 KEU/L, P < 0.01; DAO, 0.65 ± 0.12 kU/L vs 1.36 ± 0.22 kU/L, P < 0.01).
CONCLUSION: The expression of HMGB1 mRNA increases in the intestine during SAP. PDTC markedly inhibits HMGB1 mRNA gene expression.
- Citation: Luan ZG, Zhang C, Ge CL, Ma XC, Guo RX. Role of high mobility group box-1 protein in gut mucosal barrier dysfunction during severe acute pancreatitis in rats. Shijie Huaren Xiaohua Zazhi 2007; 15(30): 3173-3177
- URL: https://www.wjgnet.com/1009-3079/full/v15/i30/3173.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v15.i30.3173
重症急性胰腺炎(severe acute pancreatitis, SAP)时肠黏膜受损伴发肠道细菌和内毒素移位是胰腺炎继发感染的根源, 严重时可导致多器官功能障碍[1-4]. 近年研究发现, 高迁移率族蛋白B1(high mobility group box-1 protein, HMGB1)作为晚期炎症介质参与疾病的病理生理过程, 其表达增加与脏器功能损害密切相关[5-9]. 我们观察SAP时肠黏膜内HMGB-1的动态变化, 探讨HMGB-1在SAP肠黏膜屏障损害中的作用.
牛磺胆酸钠、二硫代氨基甲酸吡咯烷(PDTC)、DAO标准品由Sigma公司提供, 内毒素检测试剂盒购自湛江安度斯生物有限公司, RNA提取及PCR扩增试剂盒购自TaKaRa公司, PCR仪及电泳仪和凝胶扫描分析系统购自美国Kodak. 大鼠HMGB1序列(扩增片段为680 bp): 上游5'-ATGGGCAAAGGAGATCCTA-3', 下游5'-ATTCATCATCATCATCTTCT-3'[5]; 大鼠GAPDH序列(扩增片段为309 bp): 上游5'-TCCCTCAAGATTGTCAGCAA-3', 下游5'-AGATCCACAACGGATACATT-3'[10]. 羊抗HMGB1 mAb购自Santa Cruz公司, 辣根过氧化物酶标记的兔抗羊IgG购自深圳晶美公司, 健康成年♂Wistar大鼠56只(体质量270-330 g), 购自中国医科大学实验动物学部.
大鼠随机分为正常对照组(Control组, n = 8), SAP组(n = 40), PDTC组(n = 8), SAP组分为建模后3, 6, 12, 24, 48 h共5个亚组(每亚组8只). 大鼠术前12 h禁食, 不禁饮. 20 g/L戊巴比妥钠ip麻醉(1 mL/kg), 常规消毒后, 腹部正中切口入腹腔, 找到胆胰管, 于其出肝门端以动脉夹暂时阻闭胆管, 从乳头处的十二指肠壁进针, 逆行刺入胆胰管内并固定, 于胆胰管入十二指肠端用小动脉夹暂时阻闭胆胰管, 以0.1 mL/min速度匀速注入50 g/L牛磺胆酸钠(1.5 mL/kg), 注射完毕后10 min去除动脉夹, 逐层关腹. PDTC组于成模后即刻ip PDTC(100 mg/kg). 术后禁食, 自由饮水, sc生理盐水40 mL/(kg•6 h)行液体复苏. 正常对照组常规麻醉后取材, SAP组分别于模型成功后3, 6, 12, 24, 48 h取材, PDTC组于模型成功后24 h取材. 于上述时间点麻醉动物, 腹主动脉采血, 离心(3000 r/min, 15 min)分离血浆, 分装冻存于-80℃待测. 组织标本严格无菌采取, 组织置于经消毒的管, 液氮速冻, -70℃贮存备用. 血浆LPS水平测定采用动态浊度法鲎试剂检测LPS水平. 所用器具经灭菌、去热源处理. 血浆二胺氧化酶(DAO)水平测定[11-12]按改良分光光度法测定.
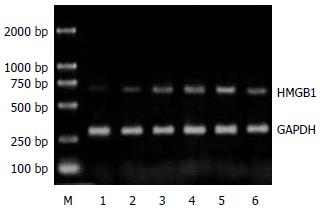
1.2.1 RT-PCR方法检测肠组织HMGB1 mRNA表达: 取小肠组织80 mg, 采用TaKaRa公司RNA提取试剂盒提取提总RNA, 用紫外分光光度计测定A260:A280比值, 该值稳定于1.8-2.0. 按试剂盒说明合成cDNA后取反转录产物, 加入PCR反应体系, 扩增目的基因. 以三磷酸甘油醛脱氢酶(GAPDH)作为内参对照, 扩增产物经15 g/L琼脂糖凝胶电泳检测, 凝胶用美国Kodak凝胶扫描分析系统测定积分光密度值, 通过目的基因与内参对照的积分光密度比值表示mRNA表达的相对含量.
1.2.2 Western blot法检测肠组织HMGB1水平: 取肠组织约0.2 g, 4℃匀浆, 加入蛋白提取液, 12 000 r/min离心20 min, 取上清液, 以考马斯亮蓝法进行蛋白定量. 再将提取的蛋白等量加样, 经SDS-PAGE电泳分离样品(40 μg)后电转移至硝酸纤维膜上. 转膜后的硝酸纤维膜经漂洗后用50 g/L脱脂奶粉阻断非特异性结合, 然后加入一抗(羊抗HMGB1mAb, Santa Cruz, 浓度1:400)4℃孵育过夜, 加入标记二抗(辣根过氧化物酶标记的兔抗羊IgG, 深圳晶美, 浓度1:1000)37℃孵育1 h, ECL浸膜显色. 在图像分析仪上对胶片进行扫描, 测定各条带的吸光度值.
统计学处理 所有数据均以mean±SD表示, 采用单因素方差分析, 经SPSS11.0统计分析软件对数据进行处理.
与正常对照组相比, SAP组发病后6 h, 大鼠血浆中LPS含量上升, 24 h达峰值, 48 h仍显著高于正常对照组(P<0.01). PDTC干预后, 大鼠血浆LPS含量显著降低(P<0.01, 表1). 在SAP组3 h时血浆DAO水平较正常对照组无明显变化, 在SAP建模后6 h时血浆DAO水平明显上升, 24 h达峰, 48 h仍显著高于对照组(P<0.01). PDTC组较SAP 24 h组血浆DAO水平明显下降(P<0.01, 表1).
| 血浆 | 肠组织 | ||||
| LPS( kEU/L) | DAO( kU/L) | HMGB1 mRNA | HMGB1 | ||
| Control | - | 0.106±0.008 | 0.32±0.02 | 0.26±0.03 | 26.3±4.3 |
| SAP | 3 | 0.121±0.103 | 0.35±0.03 | 0.32±0.05 | 30.5±5.2 |
| 6b | 0.447±0.153 | 0.68±0.14 | 0.41±0.06 | 68.4±7.4 | |
| 12b | 0.614±0.204 | 0.94±0.17 | 0.56±0.12 | 84.1±12.6 | |
| 24b | 0.852±0.232 | 1.36±0.22 | 0.62±0.06 | 132.6±32.5 | |
| 48b | 0.627±0.145 | 0.88±0.16 | 0.54±0.05 | 107.6±26.4 | |
| PDTC | 24bd | 0.433±0.120 | 0.65±0.12 | 0.35±0.06 | 70.2±9.3 |
正常对照组大鼠肠组织中HMGB1 mRNA表达微弱. SAP建模后6 h, 大鼠肠组织中HMGB1 mRNA转录水平较正常对照组增高, 12 h显著升高, 24 h达峰值并持续至48 h(P<0.01, 表1, 图1). 应用PDTC干预后, PDTC组大鼠肠组织中HMGB1 mRNA转录水平显著低于SAP 24 h组(P<0.01, 表1, 图2). 正常情况下大鼠肠组织中有少量的HMGB1表达. SAP模型制成6 h后, 大鼠肠组织中HMGB1表达开始增高, 较对照组差异显著(P<0.01); 12 h呈现进一步升高趋势, 24 h达峰值, 至48 h仍维持在较高水平(P<0.01). 给予PDTC处理后, 与SAP 24 h组相比, PDTC组大鼠肠组织中HMGB1表达明显下降(P<0.01, 表1, 图3).
重症急性胰腺炎(severe acute pancreatitis, SAP)发病凶险, 并发症多, 可在短时间内损伤多个脏器. 近年来其救治成功率有所提高, 但病死率仍为12%-15%. 有并发症者可高达到50%, 而其中80%的死亡原因与SAP患者肠道屏障功能受继发感染有关[13-15]. SAP 时肠黏膜遭到破坏, 肠绒毛萎缩, 尖端坏死, 微绒毛脱落, 肠道屏障作用的削弱使肠道内细菌及其毒素易于穿透受损的黏膜上皮向肠外移位, 成为继发性感染的根源[16-20]. HMGB1是细胞核内一种含量丰富的非组蛋白, 广泛地存在于真核细胞核中, 因在电泳时快速迁移而得名, 他与DNA复制、细胞增殖分化及基因转录等多种生命活动有关.研究发现, HMGB1可分泌到细胞外发挥致炎效应, 其产生明显晚于TNF-α, IL-1等早期炎性介质, 且持续时间较长, 与严重全身炎症反应综合征及多器官功能障碍等病理过程密切相关[21-24]. 阻断HMGB1的表达或抑制HMGB1的活性可明显减轻炎症反应和组织损伤[25]. 新近研究表明, 严重创伤或感染时, 组织HMGB1基因表达上调, HMGB1可介导肠屏障功能不全[21,26-28].
本研究结果显示, SAP时大鼠肠组织中HMGB1表达在建模后数小时内无明显变化, 于建模后6 h即上升, 24 h达峰值, 48 h仍高于正常, 提示HMGB1对肠组织有直接损伤作用, HMGB1可能参与SAP肠组织损伤的病理生理过程. PDTC能够选择性地抑制NF-κB的活化[29], 使用PDTC抑制NF-κB通路后, SAP大鼠肠组织中HMGB1表达明显下调, 提示NF-κB可能直接或间接参与HMGB1诱生的细胞信号转导过程[30-32]. 我们观察到, SAP大鼠肠组织HMGB1表达延迟并持续增高的同时, 血浆LPS、DAO等反映肠黏膜屏障功能的指标也不同程度的升高. SAP建模后6 h, 大鼠血浆中LPS含量与DAO水平明显上升, 24 h达峰值, 48 h仍显著高于正常对照组, 说明HMGB1作为晚期炎症介质可使肠黏膜损伤加重, 肠黏膜通透性持续增加. 给予PDTC干预后, HMGB1表达下调, 同时SAP大鼠血浆LPS含量与DAO水平明显下降, 说明肠黏膜屏障功能得到明显改善, 其机制可能为SAP大鼠应用PDTC抑制NF-κB活化后, 肠组织HMGB1表达受到抑制, HMGB1对肠组织的直接损伤作用减轻[26]. HMGB1与TNF-α, IL-1等早期炎性介质可相互诱导[33], NF-κB活性受抑后TNF-α, IL-1, iNOS及黏附分子等早期炎性介质表达下调进而阻断HMGB1与早期炎性介质的相互诱导, 从而改善肠黏膜屏障功能. 肠道细菌移位是SAP并发感染的主要原因, HMGB1作为晚期炎症介质在SAP肠黏膜屏障功能损害中起重要作用[34]. 通过应用PDTC可下调HMGB1水平, 能有效维护肠黏膜屏障功能, 为预防和治疗SAP继发肠黏膜屏障损害提供新途径.
SAP时肠黏膜屏障功能受到损害, 导致细菌和内毒素的移位, 从而引发肠源性感染和内毒素血症, 严重时可促使多器官功能衰竭发生. 最近研究表明, 高迁移率族蛋白B1相对于TNF-α、IL-1β等早期炎症介质分泌延迟且持续时间较长, 是一种新的"晚期"炎症介质, 能给脓毒症带来更广的"治疗窗".
HMGB1作为晚期炎症介质在SAP肠黏膜屏障功能损害中起重要作用, 通过抑制HMGB1的表达可减轻组织的炎症反应, 能有效维护肠黏膜屏障功能, 为预防和治疗SAP继发肠黏膜屏障损害切实可行的干预目标.
本文研究结果表明, HMGB1在SAP肠组织中表达较晚, 持续时间较长, 说明HMGB1在SAP肠黏膜损害中起重要作用. 应用PDTC可下调HMGB1表达水平, 能够减轻肠黏膜屏障损害, 为SAP肠黏膜屏障损害的防治提供新的思路.
HMGB1: 是一大类高度保守的蛋白质, 是细胞核内含量丰富的非组蛋白染色质蛋白, 分子量较低(30 kDa), 因其在聚丙烯酰胺凝胶电泳中迁移迅速而得名. 可参与DNA复制、细胞分化及基因表达等多种细胞生命活动. 与TNF-α和IL-1β等早期细胞因子相比, HMGB1出现较晚且持续时间更长, 可能成为脓毒症防治切实可行的干预目标.
本文立意较为新颖, 实验目的明确, 紧扣SAP这一研究热点, 实验设计较为严密, 动物模型成熟, 统计方法选择合理, 有一定的可读性.
编辑: 程剑侠 电编:李军亮
| 1. | Garside P, Millington O, Smith KM. The anatomy of mucosal immune responses. Ann N Y Acad Sci. 2004;1029:9-15. [PubMed] |
| 2. | Harari Y, Weisbrodt NW, Moody FG. Ileal mucosal response to bacterial toxin challenge. J Trauma. 2000;49:306-313. [PubMed] |
| 3. | Mifkovic A, Pindak D, Daniel I, Pechan J. Septic complications of acute pancreatitis. Bratisl Lek Listy. 2006;107:296-313. [PubMed] |
| 4. | Pearce CB, Zinkevich V, Beech I, Funjika V, Ruiz AG, Aladawi A, Duncan HD. Using the polymerase chain reaction coupled with denaturing gradient gel electrophoresis to investigate the association between bacterial translocation and systemic inflammatory response syndrome in predicted acute severe pancreatitis. World J Gastroenterol. 2005;11:7142-7147. [PubMed] |
| 5. | Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248-251. [PubMed] |
| 6. | Wang H, Yang H, Tracey KJ. Extracellular role of HMGB1 in inflammation and sepsis. J Intern Med. 2004;255:320-331. [PubMed] |
| 7. | Yang H, Wang H, Czura CJ, Tracey KJ. The cytokine activity of HMGB1. J Leukoc Biol. 2005;78:1-8. [PubMed] |
| 8. | Lutz W, Stetkiewicz J. High mobility group box 1 protein as a late-acting mediator of acute lung inflammation. Int J Occup Med Environ Health. 2004;17:245-254. [PubMed] |
| 9. | Williams LJ, Guernsey DL, Casson AG. Biomarkers in the molecular pathogenesis of esophageal (Barrett) adenocarcinoma. Curr Oncol. 2006;13:33-43. [PubMed] |
| 10. | Liu S, Adcock IM, Old RW, Barnes PJ, Evans TW. Lipopolysaccharide treatment in vivo induces widespread tissue expression of inducible nitric oxide synthase mRNA. Biochem Biophys Res Commun. 1993;196:1208-1213. [PubMed] |
| 11. | Kamei H, Hachisuka T, Nakao M, Takagi K. Quick recovery of serum diamine oxidase activity in patients undergoing total gastrectomy by oral enteral nutrition. Am J Surg. 2005;189:38-43. [PubMed] |
| 12. | Fu XB, Xing F, Yang YH, Sun TZ, Guo BC. Activation of phosphorylating-p38 mitogen-activated protein kinase and its relationship with localization of intestinal stem cells in rats after ischemia-reperfusion injury. World J Gastroenterol. 2003;9:2036-2039. [PubMed] |
| 13. | Besselink MG, van Santvoort HC, Witteman BJ, Gooszen HG. Management of severe acute pancreatitis: it's all about timing. Curr Opin Crit Care. 2007;13:200-206. [PubMed] |
| 14. | Marotta F, Geng TC, Wu CC, Barbi G. Bacterial translocation in the course of acute pancreatitis: beneficial role of nonabsorbable antibiotics and lactitol enemas. Digestion. 1996;57:446-452. [PubMed] |
| 15. | Butturini G, Salvia R, Bettini R, Falconi M, Pederzoli P, Bassi C. Infection prevention in necrotizing pancreatitis: an old challenge with new perspectives. J Hosp Infect. 2001;49:4-8. [PubMed] |
| 16. | Nettelbladt CG, Katouli M, Bark T, Svenberg T, Mollby R, Ljungqvist O. Evidence of bacterial translocation in fatal hemorrhagic pancreatitis. J Trauma. 2000;48:314-315. [PubMed] |
| 17. | Wu CT, Li ZL, Xiong DX. Relationship between enteric microecologic dysbiosis and bacterial translocation in acute necrotizing pancreatitis. World J Gastroenterol. 1998;4:242-245. [PubMed] |
| 18. | Cicalese L, Sahai A, Sileri P, Rastellini C, Subbotin V, Ford H, Lee K. Acute pancreatitis and bacterial translocation. Dig Dis Sci. 2001;46:1127-1132. [PubMed] |
| 19. | Clavel T, Haller D. Molecular interactions between bacteria, the epithelium, and the mucosal immune system in the intestinal tract: implications for chronic inflammation. Curr Issues Intest Microbiol. 2007;8:25-43. [PubMed] |
| 20. | van Minnen LP, Blom M, Timmerman HM, Visser MR, Gooszen HG, Akkermans LM. The use of animal models to study bacterial translocation during acute pancreatitis. J Gastrointest Surg. 2007;11:682-689. [PubMed] |
| 21. | Abraham E, Arcaroli J, Carmody A, Wang H, Tracey KJ. HMG-1 as a mediator of acute lung inflammation. J Immunol. 2000;165:2950-2954. [PubMed] |
| 22. | Lin X, Yang H, Sakuragi T, Hu M, Mantell LL, Hayashi S, Al-Abed Y, Tracey KJ, Ulloa L, Miller EJ. Alpha-chemokine receptor blockade reduces high mobility group box 1 protein-induced lung inflammation and injury and improves survival in sepsis. Am J Physiol Lung Cell Mol Physiol. 2005;289:L583-L590. [PubMed] |
| 23. | Angus DC, Yang L, Kong L, Kellum JA, Delude RL, Tracey KJ, Weissfeld L. Circulating high-mobility group box 1 (HMGB1) concentrations are elevated in both uncomplicated pneumonia and pneumonia with severe sepsis. Crit Care Med. 2007;35:1061-1067. [PubMed] |
| 24. | Li W, Li J, Ashok M, Wu R, Chen D, Yang L, Yang H, Tracey KJ, Wang P, Sama AE. A cardiovascular drug rescues mice from lethal sepsis by selectively attenuating a late-acting proinflammatory mediator, high mobility group box 1. J Immunol. 2007;178:3856-3864. [PubMed] |
| 25. | Silva E, Arcaroli J, He Q, Svetkauskaite D, Coldren C, Nick JA, Poch K, Park JS, Banerjee A, Abraham E. HMGB1 and LPS induce distinct patterns of gene expression and activation in neutrophils from patients with sepsis-induced acute lung injury. Intensive Care Med. 2007;33:1829-1839. [PubMed] |
| 27. | Fang WH, Yao YM, Shi ZG, Yu Y, Wu Y, Lu LR, Sheng ZY. The significance of changes in high mobility group-1 protein mRNA expression in rats after thermal injury. Shock. 2002;17:329-333. [PubMed] |
| 28. | Sappington PL, Yang R, Yang H, Tracey KJ, Delude RL, Fink MP. HMGB1 B box increases the permeability of Caco-2 enterocytic monolayers and impairs intestinal barrier function in mice. Gastroenterology. 2002;123:790-802. [PubMed] |
| 30. | Kokkola R, Andersson A, Mullins G, Ostberg T, Treutiger CJ, Arnold B, Nawroth P, Andersson U, Harris RA, Harris HE. RAGE is the major receptor for the proinflammatory activity of HMGB1 in rodent macrophages. Scand J Immunol. 2005;61:1-9. [PubMed] |
| 31. | Park JS, Svetkauskaite D, He Q, Kim JY, Strassheim D, Ishizaka A, Abraham E. Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J Biol Chem. 2004;279:7370-7377. [PubMed] |
| 32. | Park JS, Gamboni-Robertson F, He Q, Svetkauskaite D, Kim JY, Strassheim D, Sohn JW, Yamada S, Maruyama I, Banerjee A. High mobility group box 1 protein interacts with multiple Toll-like receptors. Am J Physiol Cell Physiol. 2006;290:C917-C924. [PubMed] |
| 33. | Andersson U, Wang H, Palmblad K, Aveberger AC, Bloom O, Erlandsson-Harris H, Janson A, Kokkola R, Zhang M, Yang H. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J Exp Med. 2000;192:565-570. [PubMed] |
| 34. | Sawa H, Ueda T, Takeyama Y, Yasuda T, Shinzeki M, Nakajima T, Kuroda Y. Blockade of high mobility group box-1 protein attenuates experimental severe acute pancreatitis. World J Gastroenterol. 2006;12:7666-7670. [PubMed] |