修回日期: 2007-04-11
接受日期: 2007-04-13
在线出版日期: 2007-06-18
目的: 探讨大肠癌组织中胃泌素(GAS)、生长抑素(SS)的表达及其比势与细胞周期调控蛋白P16INK4a、P21CIP1及细胞周期素(Cyclins)、细胞周期依赖性蛋白激酶(CDKs)表达的关系.
方法: 随机选择79例大肠癌患者的手术切除标本, 采用免疫组化SP法检测GAS、SS、P16INK4a、P21CIP1、Cyclin D1、Cyclin E、Cyclin A、Cyclin B1、CDK2、CDK4的表达情况.
结果: Cyclin D1、CDK2、CDK4、Cyclin A在GAS高表达组、中表达组的阳性表达率明显高于低表达组; 而P16INK4a、P21CIP1阳性表达率与此相反. Cyclin E在SS低表达组的阳性表达率明显高于中表达组、高表达组; P21CIP1在SS高表达组、中表达组的阳性表达率明显高于低表达组; CDK2在SS低表达组的阳性表达率明显高于SS高表达组. 大肠癌组织中GAS、SS表达的积分比值(GAS/SS)与Cyclin D1(r = 0.252)、Cyclin E(r = 0.387)、Cyclin A(r = 0.466)、CDK2(r = 0.519)、CDK4(r = 0.434)呈正相关(P<0.01)与P16INK4a(r = -0.385)、P21CIP1(r = -0.454)蛋白的表达积分呈负相关(P<0.01).
结论: GAS、SS对大肠癌细胞生长的调控可能与P16INK4a、P21CIP1、Cyclin D1、Cyclin A、CDK2、CDK4、Cyclin E基因异常表达有关. GAS对大肠癌细胞周期的调控位点可能在G1、S、G2期, SS对大肠癌细胞周期的调控位点可能在G1/S、G2/M期的交界点, 即S、M期的入口. 对大肠癌GAS/SS积分比值分析, 可作为临床大肠癌生物学行为的重要评估指标.
引文著录: 吴佩, 茆家定, 梁林虎, 闫竞一. 大肠癌胃泌素、生长抑素的表达及比势与细胞周期调控基因的相关性. 世界华人消化杂志 2007; 15(17): 1921-1927
Revised: April 11, 2007
Accepted: April 13, 2007
Published online: June 18, 2007
AIM: To explore correlations among the expression of gastrin (GAS), somatostatin (SS), P16INK4a, P21CIP1, Cyclins, and Cyclin-dependent-kinases (CDKs) in large intestine carcinomas.
METHODS: Seventy-nine resected large intestine carcinomatous specimens were randomly selected. GAS, SS, P16INK4a, P21CIP1, Cyclin D1, Cyclin E, Cyclin A, Cyclin B1, CDK2, and CDK4 were detected by immunohistochemistry (Streptavidin-Peroxidase, SP).
RESULTS: Positive expression rates of Cyclin D1, CDK2, CDK4, and Cyclin A were significantly higher in cases showing high or medium expression levels of GAS than in cases with low GAS expression. The positive expression rates of P16INK4a and P21CIP1 were the reverse. The positive expression rate of Cyclin E was significantly lower in the high and middle SS expression groups than in the low SS expression group. The positive expression rate of P21CIP1 was significantly higher, and that of CDK2 was significantly lower, in the high and middle SS expression groups compared with the low SS expression group. The integral GAS/SS was positively correlated with Cyclin D1 (r = 0.252), Cyclin E (r = 0.387), Cyclin A (r = 0.466), CDK2 (r = 0.519), and CDK4 (r = 0.434, P < 0.01), but negatively correlated with P16INK4a (r = -0.385) and P21CIP1 (r = -0.454, P < 0.01).
CONCLUSION: The regulation and control of GAS and SS in large intestine carcinoma cell growth may be directly related to the abnormal expression of P16INK4a, P21CIP1, Cyclin D1, Cyclin A, CDK2 CDK4, and Cyclin E. The integral GAS/SS may be considered an important evaluating target for clinical determinations of the biological behavior of large intestine carcinomas.
- Citation: Wu P, Mao JD, Liang LH, Yan JY. Correlations among gastrin and somatostatin expressions and the cell cycle: controlling genes in large intestine carcinomas. Shijie Huaren Xiaohua Zazhi 2007; 15(17): 1921-1927
- URL: https://www.wjgnet.com/1009-3079/full/v15/i17/1921.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v15.i17.1921
胃泌素(gastrin, GAS)、生长抑素(somatostatin, SS)分别通过其受体对胃肠道黏膜起着十分重要的生理调节作用, GAS、SS蛋白表达的异常与肿瘤发生有关[1-5]. 近来研究已发现大肠癌的发生与胃肠激素的异常表达有密切的关系. 如生长激素能够诱导大肠癌细胞的凋亡而促进大肠癌的生长, 胃泌素的作用与其相反[6-12]. 然而胃肠激素的大肠癌生长的调节的具体分子机制仍不清楚. 我们采用免疫组化SP法检测79例大肠癌(结肠癌、直肠癌)组织中GAS、SS、P16INK4a、P21CIP1、Cyclin D1、Cyclin E、Cyclin A、Cyclin B1、CDK2、CDK4的表达情况, 探讨大肠癌组织中GAS、SS的表达及其比势与细胞周期调控蛋白P16INK4a、P21CIP1及Cyclins、CDKs表达的相关性, 了解胃肠激素对大肠癌细胞增殖的具体调控位点.
收集2001-08/2005-10我院大肠癌手术切除新鲜标本共79例, 均经病理确诊. 其中直肠癌39例, 结肠癌40例, 女33例, 男46例, 年龄20-78(平均50.9±13.2)岁. 大体类型为溃疡型46例, 隆起型30例, 浸润型3例. 组织学类型按全国大肠癌协作组标准: 高分化癌(乳头状腺癌、管状腺癌Ⅰ)18例, 中分化癌(管状腺癌Ⅱ、黏液腺癌)41例, 低分化癌(管状腺癌Ⅲ、印戒细胞癌)13例, 未分化癌7例; Dukes分期: A期10例, B期28例, C期31例, D期10例. 组织学类型: 乳头状腺癌7例, 管状腺癌40例, 黏液腺癌14例, 印戒细胞癌11例和未分化癌7例; Dukes分期: A、B期38例, C、D期41例.
1.2.1 免疫组织化(SP法): 生物试剂均由北京中山生物技术有限公司提供, 具体步骤按试剂盒说明书操作. 检测79例大肠癌(结肠癌、直肠癌)组织中GAS、SS、P16INK4a、P21CIP1、CyclinD1、Cyclin E、Cyclin A、Cyclin B1、CDK2、CDK4的表达情况. 以正常胃窦黏膜作GAS阳性对照, 以正常的胰腺组织作SS阳性对照, 以胰腺组织作P16INK4a的阳性对照, 以乳腺癌组织作P21CIP1阳性对照, 以正常扁桃体组织作Cyclin A、Cyclin B1阳性对照, 以乳腺癌组织作Cyclin D1、Cyclin E阳性对照, 以反应性淋巴结作CDK2阳性对照, 以正常皮肤组织作CDK4阳性对照, 用PBS替代一抗作阴性对照.
1.2.2 结果判断标准: GAS、SS表达积分的计算先根据切片中的细胞质染色深浅, 细胞质无染色为0分, 浅黄色为1分, 棕黄色为2分, 棕褐色为3分; 再按切片中阳性细胞数占整个肿瘤细胞的百分数比例(取10个高倍视野, 每个高倍视野计数100个肿瘤细胞中的阳性细胞数, 计算其平均数)<5%为1分, 5%-10%为2分, 11%-20%为3分, >20%为4分. GAS、SS半定量积分按其二项积分的乘积数来表示, 其1-3分为低表达组, 4-8分为中表达组, >8分为高表达组. 表达积分的计算参照Fromowitz方法对组织中棕黄色反应产物根据其染色强度, 阳性细胞数的百分比作半定量处理. 无着色计0分, 浅黄色计1分, 棕黄色计2分, 棕褐色计3分. 阳性细胞数占整个肿瘤细胞的百分数比例(取10个高倍视野, 每个高倍视野计数100个肿瘤细胞中的阳性细胞数, 计算其平均数), 0%-5%计0分, 6%-25%计1分, 26%-50%计2分, 51%-75%计3分, >75%计4分. 以上两项相加, ≤2分为阴性(-), 3分为阳性(+), 4分为中度阳性(++), ≥5分为强阳性(+++).
统计学处理 采用χ2检验和Spearman等级相关经验. P<0.05为差异有显著性. 所有数据均使用专业统计软件包SPSS11.0进行统计分析处理.
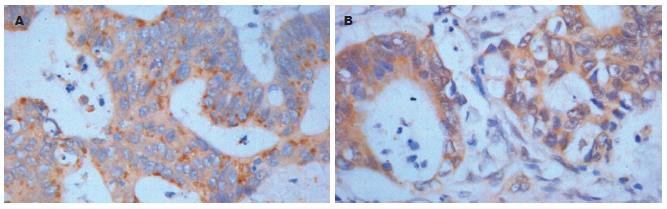
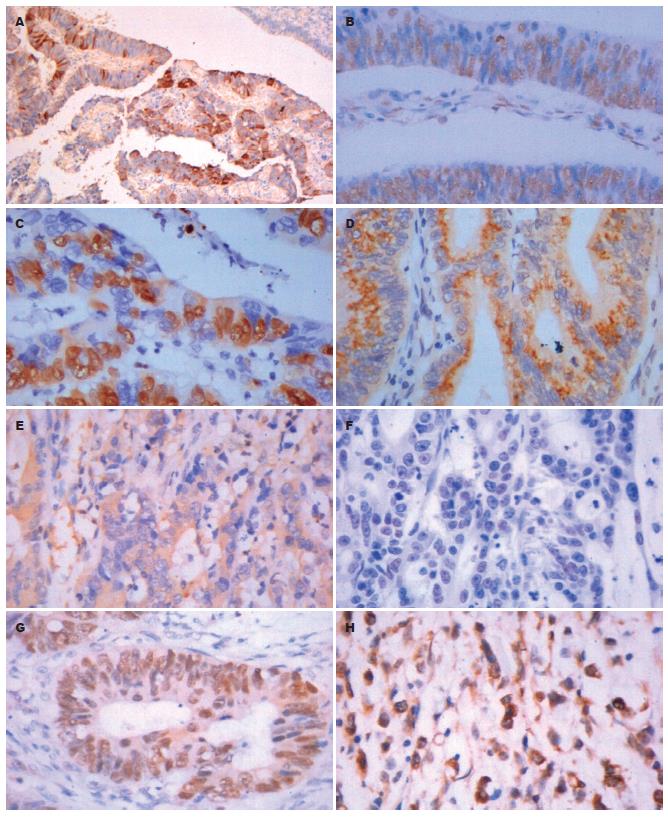
GAS、SS主要定位于细胞质, 部分定位于细胞膜上(图1). P16INK4a、P21CIP1、Cyclin D1、Cyclin E、Cyclin A、Cyclin B1、CDK2、CDK4中Cyclin B1主要定位于细胞质, P16INK4a、P21CIP1、Cyclin D1、Cyclin E、Cyclin A、CDK2、CDK4主要定位于细胞核(图2), 核内可见黄色颗粒者为阳性, 单纯细胞质着色或细胞核细胞质均无着色为阴性.
P16INK4a、P21CIP1在GAS高表达组(28.6%, 35.7%)、中表达组(34.8%, 43.5%)的阳性表达率明显低于低表达组(62.8%, 69.0%), 之间差异有显著性(χ2P16高与低 = 4.985, χ2P16中与低 = 4.719, χ2P21高与低 = 4.891, χ2P21中与低 = 4.048, 均P<0.05); Cyclin D1在GAS高表达组(78.6%)、中表达组(73.9%)的阳性表达率明显高于低表达组(45.2%), 之间差异有显著性(χ2高与低 = 4.691, χ2中与低 = 4.945, 均P<0.05); Cyclin A在GAS高表达组(100.0%)、中表达组(82.6%)的阳性表达率明显高于低表达组(54.8%)(χ2高与低 = 9.586, P<0.01; χ2中与低 = 5.040, P<0.05); CDK2在GAS高表达组(92.9%)、中表达组(87.0%)的阳性表达率明显高于低表达组(50.0%), 之间差异有显著性(χ2高与低 = 8.086, χ2中与低 = 8.715, 均P<0.01); CDK4在GAS高表达组(78.6%)、中表达组(78.3%)的阳性表达率明显高于低表达组(42.9%)(χ2高与低 = 5.364, P<0.05, χ2中与低 = 7.539, P<0.01). Cyclin E、Cyclin B1在GAS高表达组(57.1%、47.8%)、中表达组的阳性表达率(92.9%、73.9%)均高于低表达组(26.2%, 66.9%), 但三组间相比较差异无显著性(χ2 = 5.608, χ2 = 4.417, P>0.05, 表1).
P21CIP1在SS高表达组(81.8%)、中表达组(73.9%)的阳性表达率明显高于低表达组(44.4%), 之间差异有显著性(χ2高与低 = 4.945, χ2中与低 = 5.328, 均P<0.05); Cyclin E在SS低表达组的阳性表达率(53.3%)明显高于中表达组(21.7%)、高表达组(9.1%), 之间差异有显著性(χ2中与低 = 6.212, χ2高与低 = 5.325, 均P<0.05); CDK2在SS低表达组的阳性表达率(77.8%)明显高于SS高表达组(27.3%), 差异有显著 (χ2 = 8.151, P<0.01). P16INK4a在SS高表达组(63.6%)、中表达组(47.8%)的阳性表达率明显高于低表达组(46.7%), 但3组间相比较差异无显著性(χ2 = 1.049, P>0.05); Cyclin D1、CDK4在SS低表达组的阳性表达率(62.2%、64.4%)高于SS高表达组(45.5%、36.4%), 但3组间相比较差异无显著性(χ2 = 1.038, χ2 = 2.868, 均P>0.05); Cyclin A、Cyclin B1在SS高表达组(72.7%, 81.8%)、中表达组(69.6%, 73.9%)、低表达组(71.7%, 71.1%)阳性率比较差异无显著性(χ2 = 0.039, χ2 = 0.554, 均P>0.05, 表1).
GAS/SS变化与Cyclin D1、Cyclin E、Cyclin A、CDK2、CDK4半定量积分呈明显正相关(D1rs = 0.252, P<0.05; Ers = 0.387, Ars = 0.466, K2rs = 0.519, K4rs = 0.434, 均P<0.01), 与P16INK4a、P21CIP1蛋白的表达积分呈负相关(P16rs = -0.385, P21rs = -0.454, 均P<0.01); 而Cyclin B1与的积分变化无明显相关(B1rs = -0.108, P>0.05).
大肠癌的发生涉及到多个因素、多个步骤、多个阶段、多个基因突变的复杂的病理过程, 最后汇集到一个共同的环节-细胞周期机制的破坏, 故众多学者把肿瘤称为细胞周期性疾病[13-16]. 细胞周期分为G1、S、G2、M期4个连续阶段, 细胞周期的运行与否, 能否按序完成细胞周期, 受控于精密的细胞周期调控机制[17-20]. 其分子调控机制涉及细胞周期蛋白(cyclin)、细胞周期蛋白依赖激酶(cyclin-dependent kinase, CDK)以及细胞周期蛋白激酶抑制物(cyclin kinase inhibitor, CKI)3种分子的相互作用, 其中CKI分为锚蛋白家族(INK4)和双重特异性家族(CIP/KIP)两类, P16INK4a、P21CIP1分别为INK4和CIP/KIP家族的代表, 在细胞周期的调控中起负调节作用[21-26]. CDK的时相性激活是细胞周期调控机制的核心, 他主要依赖于cyclin的细胞周期特异性或时相性表达、积累与分解[27]. 人类细胞主要CDK有CDK1(CDC2)、CDK2、CDK4、CDK5、CDK6、CDK7(CAK)[28]. 细胞周期素有Cyclin B1、Cyclin A、Cyclin E、Cyclin D1、D2、D3. 而CDK与cyclin的特异性结合是细胞周期事件启动和进行的必要条件[29-32].
近年来, GAS、SS对细胞周期调控的研究有了一定的进展. 我们前期研究发现, 大肠癌组织GAS、SS的表达积分与其细胞增殖密切相关, GAS表达水平越高, 其细胞增殖活性愈高, 而SS的作用与其相反, 对细胞增殖有抑制作用[33]. Shen et al发现, GAS能促进大肠癌细胞的DNA及蛋白的合成, 而GAS的拮抗剂丙谷胺能抑制胃泌素的这一功能, 抑制大肠癌细胞从G0/G1期过渡到S期和G2, M期. Torrisani et al[34]认为胃泌素受体过表达促进肿瘤细胞增殖与P16INK4a基因突变导致对细胞周期负调节功能丧失有关. Celinski et al[35]研究发现, SS可能通过上调P16INK4a蛋白以诱导细胞周期阻滞, 抑制细胞由G1期进入S期, 从而发挥抑制肿瘤细胞生长的作用. Song et al[36]发现, G-17能诱导胃癌细胞Cyclin D1的过表达, 加快细胞从G1进入S期, 从而导致细胞失控性生长. Charland et al[37]发现, 在胰腺细胞中生长抑素能通过上调P21CIP1蛋白强烈的抑制Cyclin E的表达和CDK2的活性, 同时对Prb的磷酸化有抑制作用, 此将抑制细胞从G1期到S期的转化, 从而抑制细胞增殖. Zhao et al[38]发现, 生长抑素的类似物奥曲肽可抑制胆管癌的增殖, 他主要通过肿瘤细胞周期中G0/G1期的抑制, 而不是通过促进肿瘤细胞的凋亡来实现的, 这一细胞周期抑制效应是部分地通过上调p27kipl(CKIs机制)的表达, 下调Cyclin E-CDK2复合物的表达来实现的. 由此可见GAS、SS对消化道肿瘤细胞周期的调控位点可能与肿瘤细胞的组织来源有关. 我们的实验结果显示, GAS表达的水平越高, Cyclin D1、Cyclin A、CDK2、CDK4的阳性表达率越高, 而P16INK4a、P21CIP1越低. 此结果说明, GAS促进大肠癌细胞增殖可能是通过促进大肠癌细胞Cyclin D1、Cyclin A、CDK2、CDK4基因的过表达, 使Cyclin D1- CDK4、Cyclin A- CDK2复合物的水平增高, 进而使达到G1、S、G2期的细胞数增多, 影响细胞周期的进程, 促进细胞增殖. 此外GAS还可通过抑制大肠癌细胞P16INK4a、P21CIP1蛋白表达, 从而消弱了P16INK4a、P21CIP1基因在细胞周期G1期对CDK4的抑制作用和在G1/S、S、G2/M期对CDK2的抑制作用, 导致CDK2、CDK4过表达, 加速细胞周期G1期、S期的进程及G1/S、G2/M期转换, 促进大肠癌细胞增殖. 因此我们认为, GAS对大肠癌细胞周期的调控位点可能在G1、S、G2期. 而SS表达的水平越高, P21CIP1阳性表达率越高, Cyclin E、CDK2的阳性表达率越低. 说明SS对大肠癌细胞周期的调控一方面可能是通过促进了大肠癌细胞P21CIP1蛋白过表达, 从而增强了P21CIP1基因在细胞周期G1/S、S、G2/M期对CDK2的抑制作用, 导致CDK2呈低表达, 使细胞周期G1/S、G2/M期转换延迟及S期阻滞, 抑制细胞增殖. 另一方面可通过抑制大肠细胞Cyclin E、CDK2基因的表达, 使Cyclin E -CDK2复合物的水平降低, 进而抑制细胞从G1期到S期的转化, 使进入S期的细胞数减少, 以诱导细胞周期阻滞, 抑制细胞增殖. 因此, SS对大肠癌细胞周期的调控位点可能在G1/S、G2/M期的交界点, 即S、M期的入口.
大肠癌组织中GAS、SS比势研究发现GAS/SS比值的变化能够反映大肠癌的部分生物学特性, 如大肠癌的发生、发展及其恶性表型、分化程度、临床分期等, 其GAS/SS积分比值的升高在肿瘤发生、发展上具有更重要的意义. 本实验结果显示: GAS/SS表达积分的比值变化与Cyclin D1、Cyclin E、Cyclin A、CDK2、CDK4半定量积分呈明显正相关, 与P16INK4a、P21CIP1呈显著负相关; 提示大肠癌组织GAS、SS的异常表达, 导致大肠癌细胞周期调控因子表达失常, 可能是胃肠激素对大肠癌生长的调控机制之一. 对大肠癌GAS/SS积分比值的分析, 可作为临床大肠癌生物学行为的重要评估指标.
大肠癌是消化道最常见的恶性肿瘤之一, 发病率、死亡率均较高, 而早期诊断和根治办法尚未有突破性的进展, 10 a生存率徘徊在50%左右. 随着生活水平的不断提高, 饮食习惯的改变, 我国大肠癌的发病率日渐增高, 已跃居第3-5位. 据预测,我国大肠癌的发病率与死亡率在今后很长一段时期内将稳步上升,成为我国最常见的、发病率上升的恶性肿瘤之一. 然而, 大肠癌治疗效果却不尽理想,约有半数患者治疗失败. 因此,如何更好地干预、阻断大肠癌的发生发展是当前应迫切解决的问题, 对他开展深入的研究具有深刻的临床意义和社会意义.
人们近年来研究发现许多组织的生长受激素调节,这些组织发生的肿瘤依然受激素控制. 胃肠激素可调节胃肠黏膜细胞的增殖与分化,同时也能促进或抑制部分胃肠肿瘤的生长, 如胃泌素对胃肠黏膜细胞有营养作用, 其对大肠癌细胞的增殖具有促进作用; 而生长抑素具有广泛的抑制作用, 可抑制大肠癌的生长. 因此可以推断胃泌素、生长抑素可能会直接或间接的影响到细胞周期的调控, 从而促进或抑制大肠癌细胞的增殖.
本文层次分明, 数据可信, 图片清晰, 对基础研究与临床研究都有重要的参考价值与指导意义.
编辑: 王晓瑜 电编:郭海丽
| 1. | Gurevich L, Kazantseva I, Isakov VA, Korsakova N, Egorov A, Kubishkin V, Bulgakov G. The analysis of immunophenotype of gastrin-producing tumors of the pancreas and gastrointestinal tract. Cancer. 2003;98:1967-1976. [PubMed] |
| 2. | Cho KH, Lee HS, Ku SK. Changes in gastric endocrine cells in Balb/c mice bearing CT-26 carcinoma cells: an immunohistochemical study. Eur J Histochem. 2006;50:293-300. [PubMed] |
| 3. | Saga T, Tamaki N, Itoi K, Yamazaki T, Endo K, Watanabe G, Maruno H, Machinami R, Koizumi K, Ichikawa T. Phase III additional clinical study of 111In-pentetreotide (MP-1727): diagnosis of gastrointestinal hormone producing tumors based on the presence of somatostatin receptors. Kaku Igaku. 2003;40:185-203. [PubMed] |
| 4. | Schally AV, Szepeshazi K, Nagy A, Comaru-Schally AM, Halmos G. New approaches to therapy of cancers of the stomach, colon and pancreas based on peptide analogs. Cell Mol Life Sci. 2004;61:1042-1068. [PubMed] |
| 5. | Tejeda M, Gaal D, Hullan L, Hegymegi-Barakonyi B, Keri G. Evaluation of the antitumor efficacy of the somatostatin structural derivative TT-232 on different tumor models. Anticancer Res. 2006;26:3477-3483. [PubMed] |
| 6. | Cao J, Yu JP, Liu CH, Zhou L, Yu HG. Effects of gastrin 17 on beta-catenin/Tcf-4 pathway in Colo320WT colon cancer cells. World J Gastroenterol. 2006;12:7482-7487. [PubMed] |
| 7. | Ferrand A, Kowalski-Chauvel A, Pannequin J, Bertrand C, Fourmy D, Dufresne M, Seva C. Glycine-extended gastrin activates two independent tyrosine-kinases in upstream of p85/p110 phosphatidylinositol 3-kinase in human colonic tumour cells. World J Gastroenterol. 2006;12:1859-1864. [PubMed] |
| 8. | Ogunwobi OO, Beales IL. Glycine-extended gastrin stimulates proliferation and inhibits apoptosis in colon cancer cells via cyclo-oxygenase-independent pathways. Regul Pept. 2006;134:1-8. [PubMed] |
| 9. | Triantafillidis JK, Merikas E, Govosdis V, Konstandellou E, Cheracakis P, Barbatzas C, Tzourmakliotis D, Peros G. Increased fasting serum levels of growth hormone and gastrin in patients with gastric and large bowel cancer. Hepatogastroenterology. 2003;50 Suppl 2:cclvi-cclx. [PubMed] |
| 10. | Sadji-Ouatas Z, Lasfer M, Julien S, Feldmann G, Reyl-Desmars F. Doxorubicin and octreotide induce a 40 kDa breakdown product of p53 in human hepatoma and tumoral colon cell lines. Biochem J. 2002;364:881-885. [PubMed] |
| 11. | Watson SA, Morris TM, McWilliams DF, Harris J, Evans S, Smith A, Clarke PA. Potential role of endocrine gastrin in the colonic adenoma carcinoma sequence. Br J Cancer. 2002;87:567-573. [PubMed] |
| 12. | Mao JD, Wu P, Xia XH, Hu JQ, Huang WB, Xu GQ. Correlation between expression of gastrin, somatostatin and cell apoptosis regulation gene bcl-2/bax in large intestine carcinoma. World J Gastroenterol. 2005;11:721-725. [PubMed] |
| 14. | Arisi E, Pruneri G, Carboni N, Sambataro G, Pignataro L. Prognostic significance of P27 and cyclin D1 co-expression in laryngeal squamous cell carcinoma: possible target for novel therapeutic strategies. J Chemother. 2004;16 Suppl 5:3-6. [PubMed] |
| 15. | Kudo Y, Kitajima S, Ogawa I, Miyauchi M, Takata T. Down-regulation of Cdk inhibitor p27 in oral squamous cell carcinoma. Oral Oncol. 2005;41:105-116. [PubMed] |
| 16. | Bosco EE, Knudsen ES. RB in breast cancer: at the crossroads of tumorigenesis and treatment. Cell Cycle. 2007;6:667-671. [PubMed] |
| 17. | Tvegard T, Soltani H, Skjolberg HC, Krohn M, Nilssen EA, Kearsey SE, Grallert B, Boye E. A novel checkpoint mechanism regulating the G1/S transition. Genes Dev. 2007;21:649-654. [PubMed] |
| 18. | Joshi KS, Rathos MJ, Mahajan P, Wagh V, Shenoy S, Bhatia D, Chile S, Sivakumar M, Maier A, Fiebig HH. P276-00, a novel cyclin-dependent inhibitor induces G1-G2 arrest, shows antitumor activity on cisplatin-resistant cells and significant in vivo efficacy in tumor models. Mol Cancer Ther. 2007;6:926-934. [PubMed] |
| 19. | Yang SH, Chien CM, Chang LS, Lin SR. Involvement of c-jun N-terminal kinase in G2/M arrest and caspase-mediated apoptosis induced by cardiotoxin III (Naja naja atra) in K562 leukemia cells. Toxicon. 2007;49:966-974. [PubMed] |
| 20. | Huang X, Di Liberto M, Cunningham AF, Kang L, Cheng S, Ely S, Liou HC, Maclennan IC, Chen-Kiang S. Homeostatic cell-cycle control by BLyS: Induction of cell-cycle entry but not G1/S transition in opposition to p18INK4c and p27Kip1. Proc Natl Acad Sci U S A. 2004;101:17789-17794. [PubMed] |
| 21. | Gibson SL, Dai CY, Lee HW, DePinho RA, Gee MS, Lee WM, Furth EE, Brensinger C, Enders GH. Inhibition of colon tumor progression and angiogenesis by the Ink4a/Arf locus. Cancer Res. 2003;63:742-746. [PubMed] |
| 22. | Chai J, Charboneau AL, Betz BL, Weissman BE. Loss of the hSNF5 gene concomitantly inactivates P21CIP/WAF1 and P16INK4a activity associated with replicative senescence in A204 rhabdoid tumor cells. Cancer Res. 2005;65:10192-10198. [PubMed] |
| 23. | Allal AS, Gervaz P, Brundler MA. Cyclin D1, cyclin E, and P21 have no apparent prognostic value in anal carcinomas treated by radiotherapy with or without chemotherapy. Br J Cancer. 2004;91:1239-1244. [PubMed] |
| 24. | Zhao P, Hu YC, Talbot IC. Expressing patterns of P16 and CDK4 correlated to prognosis in colorectal carcinoma. World J Gastroenterol. 2003;9:2202-2206. [PubMed] |
| 25. | Wong NA, Mayer NJ, Anderson CE, McKenzie HC, Morris RG, Diebold J, Mayr D, Brock IW, Royds JA, Gilmour HM. Cyclin D1 and P21 in ulcerative colitis-related inflammation and epithelial neoplasia: a study of aberrant expression and underlying mechanisms. Hum Pathol. 2003;34:580-588. [PubMed] |
| 26. | Chim CS, Wong AS, Kwong YL. Epigenetic inactivation of the CIP/KIP cell-cycle control pathway in acute leukemias. Am J Hematol. 2005;80:282-287. [PubMed] |
| 27. | Kim KT, Han SY, Jeong JS. Expression of G1 cell cycle regulators in rat liver upon repeated exposure to thioacetamide. Korean J Hepatol. 2007;13:81-90. [PubMed] |
| 29. | Upadhyay D, Chang W, Wei K, Gao M, Rosen GD. Fibroblast growth factor-10 prevents H2O2-induced cell cycle arrest by regulation of G1 cyclins and cyclin dependent kinases. FEBS Lett. 2007;581:248-252. [PubMed] |
| 30. | Ferrer JL, Dupuy J, Borel F, Jacquamet L, Noel JP, Dulic V. Structural basis for the modulation of CDK-dependent/independent activity of cyclin D1. Cell Cycle. 2006;5:2760-2768. [PubMed] |
| 31. | Hsiao YC, Hsieh YS, Kuo WH, Chiou HL, Yang SF, Chiang WL, Chu SC. The tumor-growth inhibitory activity of flavanone and 2'-OH flavanone in vitro and in vivo through induction of cell cycle arrest and suppression of cyclins and CDKs. J Biomed Sci. 2007;14:107-119. [PubMed] |
| 32. | Ely S, Di Liberto M, Niesvizky R, Baughn LB, Cho HJ, Hatada EN, Knowles DM, Lane J, Chen-Kiang S. Mutually exclusive cyclin-dependent kinase 4/cyclin D1 and cyclin-dependent kinase 6/cyclin D2 pairing inactivates retinoblastoma protein and promotes cell cycle dysregulation in multiple myeloma. Cancer Res. 2005;65:11345-11353. [PubMed] |
| 34. | Torrisani J, Buscail L. Molecular pathways of pancreatic carcinogenesis. Ann Pathol. 2002;22:349-355. [PubMed] |
| 35. | Celinski SA, Fisher WE, Amaya F, Wu YQ, Yao Q, Youker KA, Li M. Somatostatin receptor gene transfer inhibits established pancreatic cancer xenografts. J Surg Res. 2003;115:41-47. [PubMed] |
| 36. | Song DH, Rana B, Wolfe JR, Crimmins G, Choi C, Albanese C, Wang TC, Pestell RG, Wolfe MM. Gastrin-induced gastric adenocarcinoma growth is mediated through cyclin D1. Am J Physiol Gastrointest Liver Physiol. 2003;285:G217-222. [PubMed] |
| 37. | Charland S, Boucher MJ, Houde M, Rivard N. Somatostatin inhibits Akt phosphorylation and cell cycle entry, but not p42/p44 mitogen-activated protein (MAP) kinase activation in normal and tumoral pancreatic acinar cells. Endocrinology. 2001;142:121-128. [PubMed] |
| 38. | Zhao B, Zhao H, Zhao N, Zhu XG. Cholangiocarcinoma cells express somatostatin receptor subtype 2 and respond to octreotide treatment. J Hepatobiliary Pancreat Surg. 2002;9:497-502. [PubMed] |