修回日期: 2007-03-01
接受日期: 2007-03-17
在线出版日期: 2007-06-08
目的: 探讨细胞色素P4502E1(CYP2E1)酶基因多态性与非酒精性脂肪性肝病(NAFLD)的关系及NAFLD的遗传学发病机制.
方法: 采用聚合酶链反应--限制性片段长度多态性分析法(PCR-RFLP)对40例非酒精性脂肪肝患者, 20例酒精性肝病患者, 20例非脂肪肝对照者, 20例正常对照者分析CYP2E1基因多态性, 并进行相关性分析.
结果: NAFLD中c2等位基因型与正常对照组(χ2 = 8.376, P = 0.004)和非脂肪肝对照组(χ2 = 6.769, P = 0.005)相比明显升高, 差异有统计学意义(P<0.01), NAFLD中基因型分布与酒精性肝病相比无明显改变, 差异无统计学意义(P = 0.896). 在NAFLD中肝脏病变程度不同, c2等位基因频率不同, 在脂肪性肝炎(P = 0.04)和肝硬化(P = 0.000)中, 差异有统计学意义(P<0.05).
结论: NAFLD与CYP2E1酶基因多态性有关, 同时也为研究NAFLD遗传易感性提供了新的思路.
引文著录: 郭艳梅, 王沁, 祁志. 细胞色素P4502E1酶与非酒精性脂肪肝的关系. 世界华人消化杂志 2007; 15(16): 1825-1829
Revised: March 1, 2007
Accepted: March 17, 2007
Published online: June 8, 2007
AIM: To investigate correlations between cytochrome P4502E1 (CYP2E1) enzyme gene polymorphisms and non-alcoholic fatty liver disease (NAFLD).
METHODS: A polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) method was used to determined polymorphisms of CYP2E1 PstⅠ and RsaⅠ sites.
RESULTS: Genotypes were separated into three: type A (homozygous for c1 gene), type B (heterozygous for c1/c2 gene), and type C (homozygous for c2 gene). In 20 healthy controls, c1 frequency was 85.0% and c2 15.0%. In 40 patients with NAFLD, c1 was 58.8% and c2 41.2%. Comparing the NAFLD group with the healthy controls, c1 was decreased and c2 increased, significantly (P = 0.004). Comparing the NAFLD group with the alcoholic liver disease group, the frequency of the c2 allele gene was not significantly different (P = 0.896). In the NAFLD group the frequency of the c2 allele gene increased with the degree of pathogenesis of liver disease (P = 0.000).
CONCLUSION: The genetic polymorphism of CYP2E1 at the PstⅠ and RsaⅠ sites is associated with susceptibility for NAFLD.
- Citation: Guo YM, Wang Q, Qi Z. Association of CYP2E1 enzymes and susceptibility for non-alcoholic liver disease. Shijie Huaren Xiaohua Zazhi 2007; 15(16): 1825-1829
- URL: https://www.wjgnet.com/1009-3079/full/v15/i16/1825.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v15.i16.1825
非酒精性脂肪性肝病(non-alcoholic fatty liver disease, NAFLD)是一类肝组织学与酒精性肝病相似[1-2], 但无过量饮酒史的临床病理综合征, 包括单纯性脂肪肝, 脂肪性肝炎和肝硬化三种病理类型. NAFLD的病因和发病机制较复杂, 迄今尚未完全明了. 文献报道遗传易感性与其发病关系密切[3]. 细胞色素P4502E1(cytochrome P4502E1, CYP2E1)是细胞色素P450的乙醇诱导形式, 在非乙醇脱氢酶氧化途径中起重要作用, 是酒精性脂肪肝的主要发病形式[4-6]. 而NAFLD与酒精性脂肪肝有相似的病理特征, 推测CYP2E1可能在NAFLD发病中起一定作用. 我们利用聚合酶链反应-限制性片段长度多态性分析法(polymerase chain reaction-restriction fragment length polymorphism, PCR-RFLP), 研究CYP2E1基因多态性与NAFLD发病的关系, 以寻找NAFLD的易感因素, 并从分子水平探讨该病的发病机制.
所有研究对象均来自2006-03-01/11-28在兰州大学第一医院消化科住院及门诊患者以及健康体检中心体检者, 均为长期(10 a以上)居住在甘肃地区无血缘关系的甘肃籍汉族人. 检测生化全项(肝功、血脂)、肝纤维化4项、肝脏B超. 非酒精性脂肪性肝病组40例, 男/女: 29/11, 平均年龄50±6.25岁, 诊断标准符合2006-03中华医学会肝病学分会通过的"非酒精性肝病诊疗指南"[7]; 并排除病毒性、药物性、自身免疫性肝病. NAFLD组根据生化全项, 肝脏B超, CT检查及肝脏穿刺活检分为单纯性脂肪肝, 脂肪性肝炎(5例为穿刺活检证实)和肝硬化(10例为穿刺活检证实)3组. NAFLD组根据伴随症状不同分为肥胖组(体质量指数: 女≥25, 男≥27)和糖尿病组, 诊断符合WHO 1999年糖尿病诊断标准[8]. 酒精性肝病组20例, 均为男性, 平均年龄51±6.43岁, 诊断标准符合2006年"酒精性肝病诊疗指南"[9]. 非脂肪肝对照组20例, 暴露因素相似, 无肝脏疾病, 男/女: 8/12, 平均年龄50±4.32岁. 健康对照组20例, 来自健康体检人群, 男/女: 10/10, 平均年龄49±5.22岁, B超、生化全项、肝纤维化4项均正常, 不吸烟、不饮酒. 空腹抽取静脉血2 mL乙二胺四乙酸防凝处理后-80℃保存备用. 聚合酶链反应(polymerase chain reaction, PCR)扩增仪, Eneamp system 2400型, 美国Perkin-Elmer公司生产. 凝胶成像系统: HOOD-SN73S100108型, BIO-RAD, 意大利米兰生产. 内切酶RsaⅠ和PstⅠ酶购自Sangon公司. 扩增CYP2E1基因的引物由Sangon公司合成. 引物1: 5'-CCAGTCGAGTCTACAT TGTCA-3'; 引物2: 5'-TTCATTCTGTCTTCTAACTGG-3'.
1.2.1 基因组DNA制备: 用血液基因组DNA抽提试剂盒按说明书提纯DNA.
1.2.2 PCR反应条件: 94℃预变性2 min后, 94℃变性1 min, 50℃退火50 s, 72℃延伸50 s, 共40个循环. 72℃延伸10 min.
1.2.3 酶切及电泳: 在检测PCR扩增产物后, 分别用限制性内切酶RsaⅠ或PstⅠ消化PCR产物, 37℃电热恒水温箱孵育18 h. 加样于25 g/L琼脂糖凝胶上, 70 V恒压条件垂直电泳45 min. 凝胶电泳成像分析系统扫描分析仪分析各基因型并拍照.
统计学处理 采用SPSS10.0软件进行t检验, χ2检验. 对群体数据作Hardy-Weinberg平衡吻合度检验.
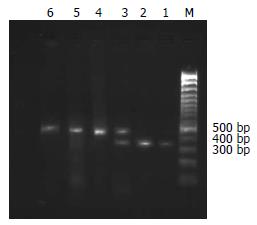
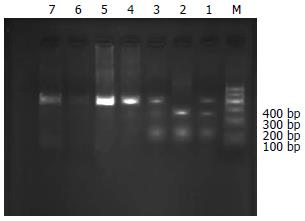
RsaⅠ的酶切位点存在-1053 C/T突变, 酶切后野生纯合子片段为360和50 bp; 杂合子为410, 360和50 bp; 突变纯合子片段为410 bp[10](图1). PstⅠ的酶切位点存在-1293 G/C突变, 酶切后野生纯合子片段为290和120 bp; 杂合子为410, 290和120 bp; 突变纯合子片段为410 bp(图2).
非酒精性脂肪性肝病组与正常对照组相比c2等位基因型明显升高, 差异有统计学意义(c2 = 8.376, P<0.05). NAFLD组与非脂肪肝对照组相比c2等位基因型明显升高, 差异有统计学意义(c2 = 6.769, P≤0.05). NAFLD组与酒精性肝病组相比, 基因型无明显改变, 差异无统计学意义(c2 = 0.017, P≥0.05, 表1).
| 分组 | n | 基因型 | 基因型 | χ2 | P | |||
| A (c1/c1) | B (c1/c2) | C (c2/c2) | c1 | c2 | ||||
| 非酒精性脂肪性肝病 | 40 | 12(30.0%) | 23(57.5%) | 5(12.5%) | 47(58.8%) | 33(41.2%) | ||
| 酒精性肝病对照 | 20 | 6(30.0%) | 12(60.0%) | 2(10.0%) | 24(60.0%) | 16(40.0%) | 0.017 | 0.896(>0.05) |
| 非脂肪肝对照 | 20 | 13(65.0%) | 7(35.0%) | 0 | 33(82.5%) | 7(17.5%) | 6.769 | 0.005(<0.05) |
| 正常对照 | 20 | 14(70.0%) | 6(30.0%) | 0 | 34(85.0%) | 6(15.0%) | 8.376 | 0.004(<0.05) |
NAFLD中, 肝脏病变程度不同, c2等位基因频率不同, 差异有统计学意义(表2).
| 分组 | n | 基因型 | 基因型 | χ2 | P | |||
| A (c1/c1) | B (c1/c2) | C (c2/c2) | c1 | c2 | ||||
| 单纯性脂肪肝 | 13 | 10(76.9%) | 3(23.1%) | 0 | 23(88.5%) | 3(11.5%) | ||
| 脂肪性肝炎 | 10 | 1(10.0%) | 8(80.0%) | 1(10.0%) | 10(50.0%) | 10(50.0%) | 8.248 | 0.04(<0.05) |
| 肝硬化 | 17 | 1(5.9%) | 12(70.6%) | 4(23.5%) | 14(41.2%) | 20(58.8%) | 13.935 | 0.000(<0.05) |
CYP2El酶是二甲基亚硝胺D-脱甲基酶, 主要在肝脏表达[11-12], 参与许多外源性化合物的代谢[13]. 大量证据表明, CYP2E1在酒精性肝病中表达明显增强[14-19], 主要参与氧应激-脂质过氧化损伤. 而非酒精性脂肪性肝病的组织病理学变化与酒精性脂肪肝相似, 推测两者在引起肝脏损害中有相似的致病机制. 动物实验证明, 在高脂饮食诱导的脂肪肝大鼠CYP2E1表达与酒精性脂肪肝相似, 即在腺泡3区表达增强, 且脂肪肝程度的加重, 表达逐渐增高[13,17,20-27]. 有研究证实在脂肪肝患者, CYP2E1的含量和活性均增加[28], 而且, 随着脂肪肝形态学上的病理严重程度增加而增加[29-30]. CYP2E1酶基因位于人第10号染色体上, 长11413个碱基对, 包括9个外显子和8个内含子[31], CYP2E1基因存在6种限制性内切酶片段长度多态性, 其中5'-端的RsaⅠ/PstⅠ多态是两个连锁多态不平衡, 存在c1单倍型(RsaⅠ+, PstⅠ-)和c2单倍型(RsaⅠ-, PstⅠ+). RsaⅠ/PstⅠ酶切位点的变异在转录水平影响CYP2E1的表达, c2位基因使其增加[32-33]. 本研究所有数据符合Hardy-Weinberg平衡吻合度检验, 说明群体基因遗传平衡, 来自同一蒙德尔群体. 研究结果发现CYP2El至少可分为3个基因型, 即A型(c1基因纯合子)、B型(c1/c2基因的杂合子)及C型(c2基因的纯合子). 在20例健康对照组中, A型为主, 说明在正常人群中以c1型为主. 在NAFLD中, 各基因型分布与正常人群相比差异有统计学意义, 这与以往研究相符[29,34]; 与酒精性脂肪肝分布相似, 以c2型为主. 说明c2型基因在NAFLD发生中起着重要的作用, 其发病机制与酒精性肝病有相似之处. 在不同伴发症NAFLD中无统计学差异, 即基因型与伴发症无关. NAFLD随肝脏病变程度增加, c2等位基因明显升高, 进一步证实c2等位基因与NAFLD的发病相关, 并更容易发展为肝硬化.
总之, CYP2E1酶基因多态性在NAFLD的发生、发展中具有重要意义, 其中c2等位基因与NAFLD的遗传易感性有关, 同时也为研究NAFLD遗传易感性提供了新的思路.
CYP2El酶是二甲基亚硝胺D-脱甲基酶, 主要在肝脏表达, 参与许多外源性化合物的代谢. 有关CYP2El酶与酒精性脂肪肝肝纤维化的关系虽有部分文献报道, 但CYP2El酶基因与非酒精性脂肪肝肝纤维化相关性的研究, 迄今国内外均少见. 关于CYP2El酶和脂肪肝易感性、肝纤维化机制的研究已成为热点.
国内外研究表明, CYP2E1在酒精性肝病中表达明显增强, 主要参与氧应激-脂质过氧化损伤. 在高脂饮食诱导的脂肪肝大鼠CYP2E1表达与酒精性脂肪肝相似, 即在腺泡3区表达增强, 且脂肪肝程度的加重, 表达逐渐增高. 在脂肪肝患者, CYP2E1的含量和活性均增加, 而且, 随着脂肪肝病理严重程度增加而增加.
在非酒精性脂肪肝中, 各基因型分布与酒精性脂肪肝分布相似, 以c2型为主. 说明c2型基因在非酒精性脂肪肝发生中起着重要的作用, 其发病机制与酒精性肝病有相似之处. 非酒精性脂肪肝随肝脏病变程度增加, c2等位基因明显升高, 进一步证实c2等位基因与非酒精性脂肪肝的发病相关, 并更容易发展为肝硬化.
CYP2E1酶基因多态性在非酒精性脂肪肝的发生、发展中具有重要作用, 其中c2等位基因与非酒精性脂肪肝的遗传易感性有关.CYP2E1可作为非酒精性脂肪肝判断肝纤维化程度和预后的指标.
本文对P4502E1的基因多态性与非酒精性脂肪肝关系进行分析, 立题新颖, 有可读性和学术价值.
编辑: 王晓瑜 电编:张敏
| 2. | Lieber CS. CYP2E1: from ASH to NASH. Hepatol Res. 2004;28:1-11. [PubMed] |
| 3. | Yu AS, Keeffe EB. Nonalcoholic fatty liver disease. Rev Gastroenterol Disord. 2002;2:11-19. [PubMed] |
| 4. | Gemma S, Vichi S, Testai E. Individual susceptibility and alcohol effects:biochemical and genetic aspects. AnnIst Super Sa. nita. 2006;42:8-16. [PubMed] |
| 5. | Zhuge J, Luo Y, Yu YN. Heterologous expression of human cytochrome P450 2E1 in HepG2 cell line. World J Gastroenterol. 2003;9:2732-2736. [PubMed] |
| 6. | Castaneda F, Rosin-Steiner S. Low concentration of ethanol induce apoptosis in HepG2 cells: role of various signal transduction pathways. Int J Med Sci. 2006;3:160-167. [PubMed] |
| 10. | Ulusoy G, Arinc E, Adali O. Genotype and allele frequencies of polymorphic CYP2E1 in the Turkish population. Arch Toxicol. 2007;10:711-718. [PubMed] |
| 11. | Pentiuk OO, Kachula SO, Herych OKh. Cytochrome P4502E1. Polymorphism, physiological function, regulation, and role in pathology. Ukr Biokhim Zh. 2004;76:16-28. [PubMed] |
| 12. | Plumlee CR, Lazaro CA, Fausto N, Polyak SJ. Effect of ethanol on innate antiviral pathways and HCV replication in human liver cells. Virol J. 2005;2:89. [PubMed] |
| 13. | Nielsen JS, Moller BL. Cloning and expression of cytochrome P450 enzymes catalyzing the conversion of tyrosine to p-hydroxyphenylacetaldoxime in the biosynthesis of cyanogenic glucosides in Triglochin maritima. Plant Physiol. 2000;122:1311-1321. [PubMed] |
| 14. | Lieber CS. Pathogenesis and treatment of alcoholic liver disease: progress over the last 50 years. RoczAkad Med Bi. alymst. 2005;50:7-20. [PubMed] |
| 15. | Wong NA, Rae F, Simpson KJ, Murray GD, Harrison DJ. Genetic polymorphisms of cytochrome p4502E1 and susceptibility to alcoholic liver disease and hepatocellular carcinoma in a white population: a study and literature review, including meta-analysis. Mol Pathol. 2000;53:88-93. [PubMed] |
| 16. | Tsutsumi M, Takada A, Wang JS. Genetic polymorphisms of cytochrome P4502E1 related to the development of alcoholic liver disease. Gastroenterology. 1994;107:1430-1435. [PubMed] |
| 17. | Lieber CS. Alcoholic fatty liver: its pathogenesis and mechanism of progression to inflammation and fibrosis. Alcohol. 2004;34:9-19. [PubMed] |
| 18. | Konishi T, Calvillo M, Leng AS, Feng J, Lee T, Lee H, Smith JL, Sial SH, Berman N, French S. The ADH3*2 and CYP2E1 c2 alleles increase the risk of alcoholism in Mexican American men. Exp Mol Pathol. 2003;74:183-189. [PubMed] |
| 19. | Leclercq IA, Farrell GC, Field J, Bell DR, Gonzalez FJ, Robertson GR. CYP2E1 and CYP4A as microsomal catalysts of lipid peroxides in murine nonalcoholic steatohepatitis. J Clin Invest. 2000;105:1067-1075. [PubMed] |
| 20. | Quertemont E. Genetic polymorphism in ethanol metabolism: acetaldehyde contribution to alcohol abuse and alcoholism. Mol Psychiatry. 2004;9:570-581. [PubMed] |
| 21. | Gonzalez FJ. Role of cytochromes P450 in chemical toxicity and oxidative stress: studies with CYP2E1. Mutat Res. 2005;569:101-110. [PubMed] |
| 22. | Rana SV, Chaudhary N, Verma Y. Circadian variation in lipid peroxidation induced by benzene in rats. Indian J Exp Biol. 2007;45:253-257. [PubMed] |
| 23. | Xu Y, Leo MA, Lieber CS. DLPC attenuates alcohol-induced cytotoxicity in HepG2 cells expressing CYP2E1. Alcohol Alcohol. 2005;40:172-175. [PubMed] |
| 24. | Hayashi N, Tsutsumi M, Fukura M, Yano H, Tsuchishima M, Takase S. Effect of chronic dietary ethanol consumption on colonic cancer in rats induced by 1,1-dimethylhydrazine. Alcohol Clin Exp Res. 2007;31:S72-76. [PubMed] |
| 25. | Kim BJ, Hood BL, Aragon RA, Hardwick JP, Conrads TP, Veenstra TD, Song BJ. Increased oxidation and degradation of cytosolic proteins in alcohol-exposed mouse liver and hepatoma cells. Proteomics. 2006;6:1250-1260. [PubMed] |
| 26. | Zhou Z, Wang L, Song Z, Saari JT, McClain CJ, Kang YJ. Zinc supplementation prevents alcoholic liver injury in mice through attenuation of oxidative stress. Am J Pathol. 2005;166:1681-1690. [PubMed] |
| 27. | Arsov T, Larter CZ, Nolan CJ, Petrovsky N, Goodnow CC, Teoh NC, Yeh MM, Farrell GC. Adaptive failure to high-fat diet characterizes steatohepatitis in Alms1 mutant mice. Biochem Biophys Res Commun. 2006;342:1152-1159. [PubMed] |
| 28. | Wan J, Ernstgard L, Song BJ, Shoaf SE. Chlorzoxa-zone metabolism is increased in fasted Sprague-Dawley rats. J Pharm Pharmacol. 2006;58:51-61. [PubMed] |
| 29. | Orellana M, Rodrigo R, Varela N, Araya J, Poniachik J, Csendes A, Smok G, Videla LA. Relationship between in vivo chlorzoxazone hydroxylation, hepatic cytochrome P450 2E1 content and liver injury in obese non-alcoholic fatty liver disease patients. Hepatol Res. 2006;34:57-63. [PubMed] |
| 30. | Wu D, Cederbaum A. Nitric oxide donors prevent while the nitric oxide synthase inhibitor L-NAME increases arachidonic acid plus CYP2E1-dependent toxicity. Toxicol Appl Pharmacol. 2006;216:282-292. [PubMed] |
| 31. | Lieber CS, Leo MA, Mak KM, Xu Y, Cao Q, Ren C, Ponomarenko A, DeCarli LM. Model of nonalcoholic steatohepatitis. Am J Clin Nutr. 2004;79:502-509. [PubMed] |
| 32. | Orellana M, Guajardo V. Cytochrome P450 activity and its alteration in different diseases. Rev Med Chil. 2004;132:85-94. [PubMed] |
| 33. | Chelule PK, Pegoraro RJ, Gqaleni N, Dutton MF. The frequency of cytochrome P450 2E1 polymorphisms in Black South Africans. Dis Markers. 2006;22:351-354. [PubMed] |
| 34. | Piao YF, Li JT, Shi Y. Relationship between genetic polymorphism of cytochrome P450IIE1 and fatty liver. World J Gastroenterol. 2003;9:2612-2615. [PubMed] |