修回日期: 2007-03-15
接受日期: 2007-03-31
在线出版日期: 2007-05-28
目的: 探讨正常胃黏膜、不典型增生胃黏膜及胃癌组织中Caveolin-1, nm23及MMP-2蛋白的表达.
方法: 应用免疫组化S-P法检测25例正常胃黏膜, 65例不典型增生胃黏膜及71例胃癌组织中的Caveolin-1, nm23及MMP-2蛋白的表达.
结果: 正常胃黏膜、不典型增生胃黏膜及胃癌组织中, Caveolin-1和nm23阳性率有递减趋势, 组间差异性有统计学意义(χ2 = 106.303, P<0.05; χ2 = 64.254, P<0.05); MMP-2蛋白阳性表达率有递增趋势, 组间差异性有统计学意义(χ2 = 35.247, P<0.05). Fisher精确概率检验显示在不同的浸润深度和淋巴结转移Caveolin-1, nm23及MMP-2阳性表达率组内差异有统计学意义(P<0.05), 而在年龄、性别和脉管侵犯组内差异无统计学意义(P>0.05). Spearman等级相关分析显示Caveolin-1与nm23表达呈正相关(r = 0.957, P<0.05), Caveolin-1与MMP-2表达呈负相关(r = -0.975, P<0.05), nm23与MMP-2表达呈负相关(r = -0.987, P<0.05).
结论: Caveolin-1与nm23的缺失以及MMP-2的过表达可能是胃癌发生、发展以及浸润转移的重要原因之一, Caveolin-1可以作为一种候选抑癌基因.
引文著录: 杨育生, 刘斌, 邢传平, 高自芳, 顾立萍, 钱震, 董亮, 苏勤军. 胃癌组织中Caveolin-1, nm23及MMP-2的表达及其意义. 世界华人消化杂志 2007; 15(15): 1725-1730
Revised: March 15, 2007
Accepted: March 31, 2007
Published online: May 28, 2007
AIM: To investigate the protein expression of Caveolin-1, nm23 and matrix metalloproteinase-2 (MMP-2) in normal gastric mucosa, gastric atypical hyperplasia and gastric carcinoma tissues.
METHODS: S-P immunohistochemical method was used to detect the expression of Caveolin-1, nm23 and MMP-2 proteins in normal gastric mucosa (n = 25), gastric atypical hyperplasia (n = 65), and gastric carcinomas (n = 71).
RESULTS: Caveolin-1 and nm23 expression showed a decreasing tendency in normal gastric mucosa, atypical hyperplasia and gastric cancer tissues ordinarily, and there were statistical differences between groups (χ2 = 106.303, P < 0.05; χ2 = 64.254, P < 0.05). However, MMP-2 expression exhibited a increasing tendency (χ2 = 35.247, P < 0.05). Fisher's exact test demonstrated that the expression of Caveolin-1, nm23 and MMP-2 protein were significantly different between the cases with different invasion depths or lymph node metastases (P < 0.05), but not between the cases with different ages, sex and vessel invasions (P > 0.05). Spearman rank correlation analysis indicated that the expression of Caveolin-1 was positively correlated with that of nm23 (r = 0.957, P < 0.05) , but negatively correlated with that of MMP-2 (r = -0.975, P < 0.05). Furthermore, there was a negative correlation between nm23 and MMP-2 expression (r = -0.987, P < 0.05).
CONCLUSION: The absent expression of Caveolin-1 and nm23 together with over-expression of MMP-2 may be one of the important causes for the onset, development and progression of gastric carcinoma. Caveolin-1 may serve as a tumor suppressor gene.
- Citation: Yang YS, Liu B, Xing CP, Gao ZF, Gu LP, Qian Z, Dong L, Su QJ. Expressions and significances of Caveolin-1, nm23 and matrix metalloproteinase -2 in gastric carcinomas. Shijie Huaren Xiaohua Zazhi 2007; 15(15): 1725-1730
- URL: https://www.wjgnet.com/1009-3079/full/v15/i15/1725.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v15.i15.1725
胃癌是我国一种常见的恶性肿瘤, 其发病率约占全身恶性肿瘤的15%-25%. 早期胃癌行根治手术, 治愈率可达86%以上; 中晚期患者5年生存率为20%左右. 死亡者中有半数于5年内死于局部复发, 45%死于转移. 转移复发有一定的过程, 在此阶段对其进行有效的治疗, 可使患者生存期延长.
Caveolin-1是细胞膜上的一种支架蛋白, 参与调控并浓缩特异脂质(胆固醇和鞘磷脂)以及修饰信号传导分子(src样激酶、H-Ras、eNOS、G蛋白)等. Caveolin-1由Clenney于1989年发现, 相对分子质量是21-24 kDa. 1999年, Engelman et al[1]证实, 该基因定位于人类染色体7q31.1, 广泛存在于多种类型的细胞中, 甚至在中枢神经、胎盘组织中也有Caveolin-1的表
达[2-3]. Caveolin-1通过磷酸化和/或去磷酸化等途径与信号分子相互作用, 调控肿瘤细胞的增殖、凋亡、黏附及运动. 目前对于Caveolin-1的认识还很少, 其在肿瘤的发生过程中机制也不十分清楚. 大多数实验支持Caveolin-1为候选的抑癌基因, 但实验结果也不完全相同. 也有研究认为是由于基因突变[4]、杂合性缺失以及过甲基化[5]等原因. Caveolin-1在结肠癌、卵巢癌、肺癌等肿瘤组织中低表达, 而在乳腺癌、前列腺、膀胱癌、食管癌组织却高表达. 另外, Caveolin-1能够抑制癌细胞的浸润和转移, 促进癌细胞的转化.
目前流行病学和病理学等方面的研究显示, 胃癌多是由胃黏膜不典型增生癌变而来. 本课题利用组织芯片技术构建胃黏膜不同病变的组织芯片, 应用免疫组化SP法对Caveolin-1, nm23和MMP-2表达的相关性进行分析, 从而探讨Caveolin-1与胃癌临床病理参数间的关系, 及其在胃癌发生、发展、浸润转移中的作用, 以其为胃癌的临床诊断及预后提供客观的实验依据.
65例不典型增生及71例胃癌标本均来自兰州军区总医院病理科2004-08/2005-07手术及存档石蜡包埋标本. 胃癌组包括男49例, 女22例, 年龄30-84(中位年龄61)岁. 所有标本术前均未行放化疗, 胃癌组织包括高分化腺癌21例, 中分化腺癌24例, 低分化腺癌26例; 其中淋巴结转移阳性组35例, 淋巴结转移阴性组36例; 有脉管侵犯者49例, 无脉管侵犯者22例. 同时取距离肿瘤组织>5 cm的胃黏膜25例作为正常黏膜. 所有标本均经40 g/L甲醛固定, 常规石蜡包埋, 4 μm厚连续切片, 分别进行HE和免疫组化染色. Caveolin-1抗体为鼠抗人mAb(稀释度为1∶400)购于美国基因公司. 即用型nm23及MMP-2鼠抗人mAb和免疫组化试剂盒购于北京中杉金桥生物技术公司. 超敏SP(鼠/兔)试剂盒(UitraSensitive TM SP, Mouse/Rabbit)购于北京中杉公司, 产品编号KIT-0689, 包括以下试剂: 内源性过氧化酶阻断剂(试剂A); 正常动物非免疫血清(试剂B); 生物素标记的第二抗体(试剂C); 链霉菌抗生物素蛋白-过氧化酶(试剂D). DAB显色液购于福州迈新生物技术开发公司.
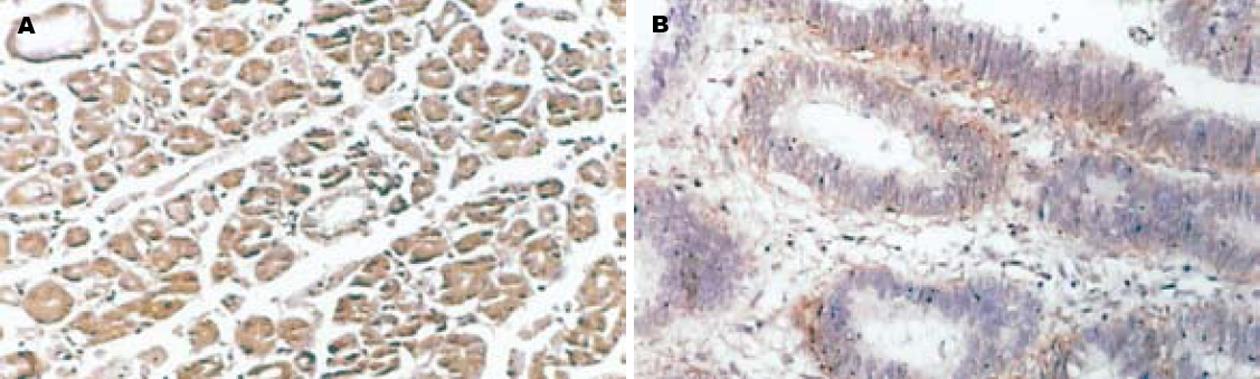
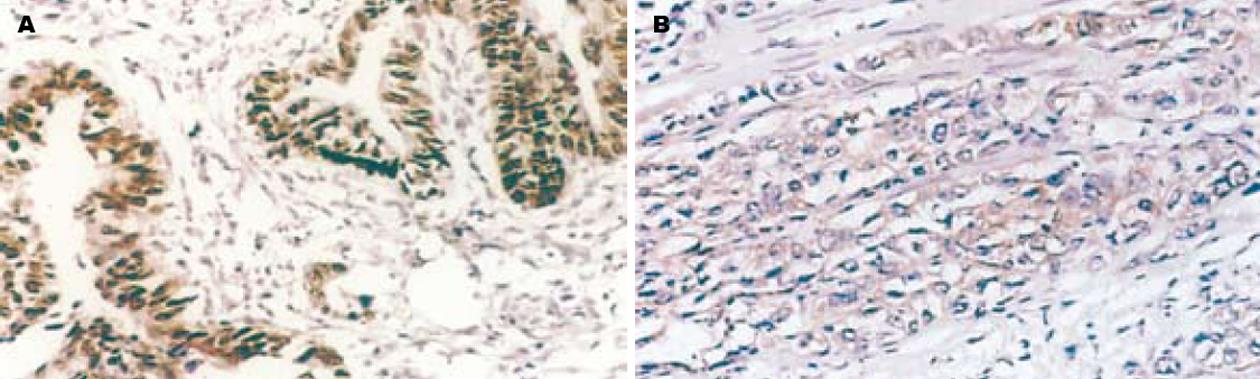
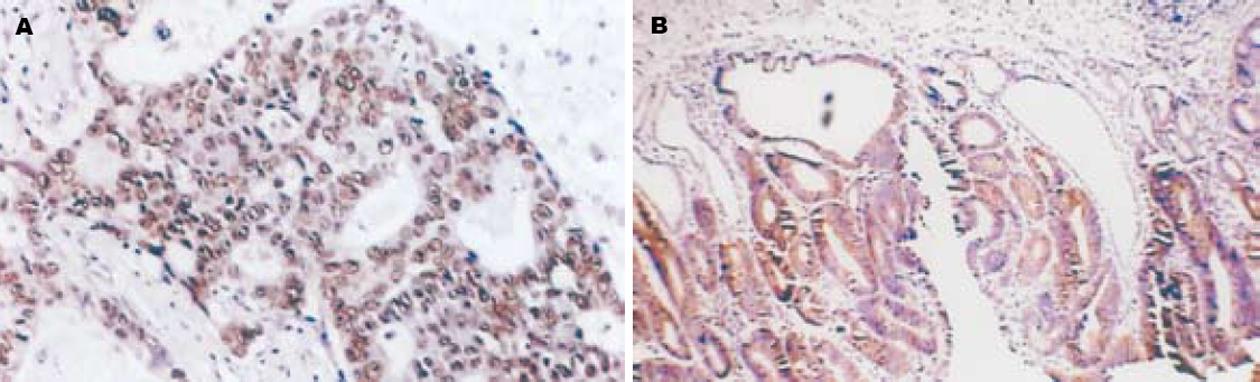
采用免疫组织化学SP法检测Caveolin-1, nm23及MMP-2在胃正常黏膜及肿瘤组织中的表达. 操作按照超敏SP(鼠/兔)试剂盒所附说明书进行, 用已知阳性片(北京中杉金桥生物技术公司提供)作阳性对照, 同时用PBS替代第一抗体作阴性对照. 结果判定: Caveolin-1, nm23及MMP-2阳性表达均位于细胞质内. 采用双盲法对每张切片在高倍镜(×400)下计数10个视野, 以染色强度分别计0-3分, 无染色为0分, 棕黄色为2分, 棕褐色为3分, 再以阳性细胞所占百分比评分, 阴性为0分, 阳性细胞≤10%为1分, 11%-50%为2分, 51%-75%为3分, >75%为4分, 两项得分相加, 0分为"-", 1-2分为"+", 3-4分为"++", 5-6分为"+++", 0分为阴性, 1-6分为阳性.
统计学处理 采用χ2检验、Fisher精确概率法和Spearman等级相关分析, 使用SPSS10.0统计软件包进行统计学处理.
Caveolin-1, nm23在正常胃黏膜、不典型增生胃黏膜及胃癌细胞胞质中有阳性表达(图1-2), χ2检验显示在正常胃黏膜、不典型增生胃黏膜及胃癌组间阳性表达率的差异性有统计学意义(χ2 = 106.303, P<0.05; χ2 = 64.254, P<0.05); MMP-2在正常胃黏膜及不典型增生胃黏膜腺上皮细胞胞质中为阴性表达, 而在胃癌细胞质中基本为阳性表达(图3), 其阳性表达率在正常胃黏膜、不典型增生胃黏膜及胃癌各组间呈递增趋势, χ2检验显示组间阳性率差异性有统计学意义(χ2 = 35.247, P<0.05)(表1).
经χ2检验和Fisher精确概率法检验, Caveolin-1, nm23及MMP-2蛋白表达在不同浸润深度、有无淋巴结转移及有无脉管侵犯组内表达率的差异有统计学意义(P<0.05), 在年龄及性别组内表达率的差异性无统计学意义(P>0.05)(表2).
| 临床病理指标 | n | Caveolin-1 | nm23 | MMP-2 | |
| 年龄(岁) | <45 | 31 | 7(22.6) | 11(35.5) | 23(74.2) |
| ≥45 | 40 | 10(25.0) | 9(22.5) | 24(60.0) | |
| 性别 | 男 | 49 | 11(22.4) | 15(30.6) | 39(79.6) |
| 女 | 22 | 6(27.3) | 5(22.7) | 18(81.8) | |
| 浸润深度 | 黏膜内 | 19 | 9(47.4)a | 11(57.9)a | 6(31.6)a |
| 肌层 | 24 | 6(25.0) | 5(20.8) | 23(95.8) | |
| 浆膜外 | 28 | 2(7.1) | 4(14.3) | 28(100) | |
| 淋巴结转移 | 无 | 35 | 14(40.0)a | 16(45.7)a | 22(62.9)a |
| 有 | 36 | 3(8.3) | 4(11.1) | 35(97.2) | |
| 脉管侵犯 | 无 | 49 | 10(20.4)a | 8(16.3)a | 38(77.6)a |
| 有 | 22 | 7(31.8) | 2(9.1) | 19(86.4) |
Spearman等级相关分析显示Caveolin-1及nm23蛋白表达呈正相关(r = 0.957, P<0.05); nm23和MMP-2蛋白表达呈负相关(r = -0.975, P<0.05); Caveolin-1和MMP-2蛋白表达呈负相关(r = -0.987, P<0.001).
Caveolin-1是Caveolae膜结构标记性蛋白, 参与调控并浓缩特异脂质(胆固醇和鞘磷脂)、细胞增殖以及修饰信号传导分子(src样激酶、H-Ras、eNOS、G蛋白)等. Caveolin-1由Clenney于1989年发现, 相对分子质量是21-24 kDa, 定位于人类染色体7q31.1, Caveolin-1通过磷酸化和/或去磷酸化等途径与信号分子相互作用, 调控肿瘤细胞的增殖、凋亡、黏附及运动[6]. 有研究认为, 由于基因突变、杂合性缺失以及过甲基化等原因, 使Caveolin-1在结肠癌[7]、卵巢癌[8]、肺癌[9]、肉瘤[10]等肿瘤组织中低表达, 而在乳腺癌[11]、前列腺[12]、膀胱癌[13]、食管癌[14]、胰腺癌[15]组织却高表达, 另外, Caveolin-1能够抑制癌细胞的浸润和转移, 促进癌细胞的转化[16-17]. Gao et al[18]发现Caveolin-1在胃癌组织中呈低表达. 本研究通过对正常胃黏膜、不典型增生胃黏膜及胃癌中Caveolin-1蛋白表达率的统计分析, 显示Caveolin-1在正常胃黏膜, 不典型增生胃黏膜, 胃癌中阳性表达率依次降低, 且组间差异性有统计学意义; 在胃癌不同的浸润深度及有无淋巴结转移组内差异性有统计学意义. 这表明Caveolin-1具有抑制胃癌发生、发展、浸润转移的作用. 另外, 本研究对Caveolin-1与抑癌基因nm23和癌基因MMP-2表达的相关性分析显示, Caveolin-1与nm23阳性表达率正相关, 与MMP-2负相关, 进一步证明Caveolin-1具有抑制胃癌发生、发展、浸润转移作用.
nm23是1983年Salerno et al[19]分离出的一种与恶性肿瘤转移有关的基因, 是目前肿瘤转移抑制基因的代表, 定位于17号染色体长臂, 参与微管聚合酶的活化和G蛋白的信号传递, 其活性与肿瘤增生过程及转移有关[20]. 低转移细胞株较高转移细胞株中的表达高约10倍. 一般认为nm23表达在多种人类肿瘤中与淋巴结转移有相关性, 而与组织学分级的关系各家报道不一[21-24]. 本研究发现, 随胃癌分化程度的降低, nm23的表达减少; 随胃癌浸润深度增加, nm23表达减少; 伴有淋巴结转移的胃癌, nm23的表达减少. 提示nm23蛋白阳性表达率越低, 胃癌的恶性程度越高, 越容易发生浸润和淋巴结转移, 与刘茗露 et al[25]报道基本一致. 可以认为nm23是与胃癌分化、浸润转移相关的抑制基因.
MMP-2是一种锌离子依赖的蛋白酶, 相对分子质量为72 kDa. MMP-2以酶原形式分泌, 不具有酶活性, Ⅰ型膜型MMP(membrane type Ⅰ MMP, MT1-MMP)等可将其激活, 被激活后的MMP-2, 能水解细胞基底膜及胞外基质中的Ⅳ型、Ⅴ型胶原和纤维连接蛋白成分, 导致基底膜破坏, 肿瘤细胞浸润结缔组织基质, 侵入小血管和淋巴管而发生转移[26]. 关于MMP-2与肿瘤浸润、转移的关系国内外作了大量的实验研究, 如Poulsom et al[27]用原位杂交技术揭示结肠癌间质细胞能合成MMP-2, 并与癌细胞共同参与浸润癌特异性组织重塑和基底膜降解过程. 本研究分析了MMP-2在胃癌中的表达情况, 发现随癌组织分化程度的降低以及浸润深度的增加, MMP-2的表达有增高的趋势; 伴有淋巴结转移的病例中, MMP-2的表达高于无淋巴结转移的病例组. 说明MMP-2在胃癌的发展、浸润和转移过程中发挥了重要作用. 这与国外学者 Sundblad[28], Wu[29], Feng et al[30]的研究结果一致.
本研究揭示胃癌组织中抑癌基因及癌基因表达产物间可能组成了复杂的肿瘤抑制及促进网络, 任何一种蛋白的功能异常, 均可使肿瘤细胞获得自主生长优势, 促进恶性肿瘤细胞的浸润和转移. 对胃癌患者进行Caveolin-1蛋白检测有可能成为临床判断胃癌分化程度、浸润深度及转移潜能的一项重要指标.
Caveolin-1是细胞质膜Caveolae(小凹)的表面标记蛋白, 是各种信号分子的支架蛋白和负向调节蛋白. Caveolin-1在信号传导、胆固醇运输、细胞内化及肿瘤抑制、肌细胞合成等方面都具有重要意义. 目前已知Caveolin-1参与了细胞增殖、分化、迁移、凋亡以及血管生成等的信号通路的调控, 从而影响肿瘤的发生、发展, 因而被认为是一种侯选的肿瘤抑制基因. 目前对于Caveolin-1基因与肿瘤发病机制之间的关系还不是很清楚(在胃癌方面的报道尚少).
本文通过探讨正常胃黏膜、不典型增生胃黏膜及胃癌组织中的抑癌基因, 及基质金属蛋白酶MMP-2的表达, 揭示了对胃癌患者进行Caveolin-1蛋白检测的重要性.
电编: 张敏 编辑:张焕兰
| 1. | Engelman JA, Zhang XL, Lisanti MP. Sequence and detailed organization of the human caveolin-1 and -2 genes located near the D7S522 locus (7q31.1). Methylation of a CpG island in the 5' promoter region of the caveolin-1 gene in human breast cancer cell lines. FEBS Lett. 1999;448:221-230. [PubMed] [DOI] |
| 2. | Virgintino D, Robertson D, Errede M, Benagiano V, Tauer U, Roncali L, Bertossi M. Expression of caveolin-1 in human brain microvessels. Neuroscience. 2002;115:145-152. [PubMed] [DOI] |
| 3. | Lyden TW, Anderson CL, Robinson JM. The endothelium but not the syncytiotrophoblast of human placenta expresses caveolae. Placenta. 2002;23:640-652. [PubMed] [DOI] |
| 4. | Lee H, Park DS, Razani B, Russell RG, Pestell RG, Lisanti MP. Caveolin-1 mutations (P132L and null) and the pathogenesis of breast cancer: caveolin-1 (P132L) behaves in a dominant-negative manner and caveolin-1 (-/-) null mice show mammary epithelial cell hyperplasia. Am J Pathol. 2002;161:1357-1369. [PubMed] [DOI] |
| 5. | Lee TS, Kim JW, Kang GH, Park NH, Song YS, Kang SB, Lee HP. DNA hypomethylation of CAGE promotors in squamous cell carcinoma of uterine cervix. Ann N Y Acad Sci. 2006;1091:218-224. [PubMed] [DOI] |
| 7. | Bender FC, Reymond MA, Bron C, Quest AF. Caveolin-1 levels are down-regulated in human colon tumors, and ectopic expression of caveolin-1 in colon carcinoma cell lines reduces cell tumorige-nicity. Cancer Res. 2000;60:5870-5878. [PubMed] |
| 8. | Bagnoli M, Tomassetti A, Figini M, Flati S, Dolo V, Canevari S, Miotti S. Downmodulation of caveolin-1 expression in human ovarian carcinoma is directly related to alpha-folate receptor overexpression. Oncogene. 2000;19:4754-4763. [PubMed] [DOI] |
| 9. | Yu JH, Wei Q, Qi FJ, Xu HT, Wang EH. Significance of caveolin-1 expression in primary lung cancer. Zhonghua Bing Li Xue Za Zhi. 2006;35:664-668. [PubMed] |
| 10. | Wiechen K, Sers C, Agoulnik A, Arlt K, Dietel M, Schlag PM, Schneider U. Down-regulation of caveolin-1, a candidate tumor suppressor gene, in sarcomas. Am J Pathol. 2001;158:833-839. [PubMed] [DOI] |
| 11. | Savage K, Lambros MB, Robertson D, Jones RL, Jones C, Mackay A, James M, Hornick JL, Pereira EM, Milanezi F. Caveolin 1 is overexpressed and amplified in a subset of basal-like and metaplastic breast carcinomas: a morphologic, ultrastructural, immunohistochemical, and in situ hybridization analysis. Clin Cancer Res. 2007;13:90-101. [PubMed] [DOI] |
| 12. | Satoh T, Yang G, Egawa S, Addai J, Frolov A, Kuwao S, Timme TL, Baba S, Thompson TC. Caveolin-1 expression is a predictor of recurrence-free survival in pT2N0 prostate carcinoma diagnosed in Japanese patients. Cancer. 2003;97:1225-1233. [PubMed] [DOI] |
| 13. | Fong A, Garcia E, Gwynn L, Lisanti MP, Fazzari MJ, Li M. Expression of caveolin-1 and caveolin-2 in urothelial carcinoma of the urinary bladder correlates with tumor grade and squamous differentiation. Am J Clin Pathol. 2003;120:93-100. [PubMed] [DOI] |
| 14. | Kato K, Hida Y, Miyamoto M, Hashida H, Shinohara T, Itoh T, Okushiba S, Kondo S, Katoh H. Overexpression of caveolin-1 in esophageal squamous cell carcinoma correlates with lymph node metastasis and pathologic stage. Cancer. 2002;94:929-933. [PubMed] [DOI] |
| 15. | Suzuoki M, Miyamoto M, Kato K, Hiraoka K, Oshikiri T, Nakakubo Y, Fukunaga A, Shichinohe T, Shinohara T, Itoh T. Impact of caveolin-1 expression on prognosis of pancreatic ductal adenocarcinoma. Br J Cancer. 2002;87:1140-1144. [PubMed] [DOI] |
| 16. | Fang K, Fu W, Beardsley AR, Sun X, Lisanti MP, Liu J. Overexpression of caveolin-1 inhibits endothelial cell proliferation by arresting the cell cycle at G0/G1 phase. Cell Cycle. 2007;6:199-204. [PubMed] [DOI] |
| 17. | Lin MI, Yu J, Murata T, Sessa WC. Caveolin-1-deficient mice have increased tumor microvascular permeability, angiogenesis, and growth. Cancer Res. 2007;67:2849-2856. [PubMed] [DOI] |
| 18. | Gao X, Sun Y, Huang L, Chen XY, Zhang KL, Kong QY, Liu J, Li H. Down-regulation of caveolin-1 in gastric carcinoma and its clinical biological significance. Ai Zheng. 2005;24:311-316. [PubMed] |
| 19. | Salerno M, Ouatas T, Palmieri D, Steeg PS. Inhibition of signal transduction by the nm23 metastasis suppressor: possible mechanisms. Clin Exp Metastasis. 2003;20:3-10. [PubMed] [DOI] |
| 20. | Delektorskaia VV, Perevoshchikov AG, Kushlinskii NE. Expression of nm23 and c-erbB-2 proteins in cells of primary colorectal cancer and its metastases. Arkh Patol. 2003;65:11-15. [PubMed] |
| 21. | Chen JQ, Zhan WH, He YL, Peng JS, Wang JP, Cai SR, Ma JP. Expression of heparanase gene, CD44v6, MMP-7 and nm23 protein and their relationship with the invasion and metastasis of gastric carcinomas. World J Gastroenterol. 2004;10:776-782. [PubMed] |
| 22. | Yu GZ, Wang JJ, Chen Y, Ni CR, Zhu MH. Expressions of nm23, P53 and S100A4 proteins and their relationships with metastasis potential in gastric carcinoma. Zhonghua Wei Chang Wai Ke Za Zhi. 2006;9:165-169. [PubMed] |
| 23. | He SQ, Zhang WY. Relationship between the expressions of KaI1, nm23, ETS-1, VEGF and microvascular density and clinical significance in nasopharyngeal carcinoma. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2006;41:813-817. [PubMed] |
| 24. | Li Y, Zhang JH, Kuang G, Yang JQ, Zhao Q, Wang XL, Jiao ZK, Zhang ZD, Wang LL. Expression of MUC1, CD44v6, nm23 in gastric carcinomas and regional lymph node tissues and their association with invasion, metastasis, and prognosis of the tumor. Ai Zheng. 2003;22:985-989. [PubMed] |
| 26. | Samantaray S, Sharma R, Chattopadhyaya TK, Gupta SD, Ralhan R. Increased expression of MMP-2 and MMP-9 in esophageal squamous cell carcinoma. J Cancer Res Clin Oncol. 2004;130:37-44. [PubMed] [DOI] |
| 27. | Poulsom R, Pignatelli M, Stetler-Stevenson WG, Liotta LA, Wright PA, Jeffery RE, Longcroft JM, Rogers L, Stamp GW. Stromal expression of 72 kda type IV collagenase (MMP-2) and TIMP-2 mRNAs in colorectal neoplasia. Am J Pathol. 1992;141:389-396. [PubMed] |
| 28. | Sundblad A, Ricci L. MMP-2 expression (type IV collagenase) in gastric cancer. Acta Gastroenterol Latinoam. 1998;28:287-290. [PubMed] |
| 29. | Wu ZY, Li JH, Zhan WH, He YL. Lymph node micrometastasis and its correlation with MMP-2 expression in gastric carcinoma. World J Gastroenterol. 2006;12:2941-2944. [PubMed] [DOI] |
| 30. | Feng G, Tan Y. Expression and significance of MMP2 and type IV collagen in gastric cancer. Zhonghua Wai Ke Za Zhi. 2000;38:775-777. [PubMed] |