修回日期: 2007-01-05
接受日期: 2007-01-27
在线出版日期: 2007-04-18
目的: 探讨肿瘤坏死因子α(TNF-α)对胃癌细胞基质金属蛋白酶2(MMP-2)和基质金属蛋白酶9(MMP-9)表达的诱导作用.
方法: 以外源性TNF-α作用于胃癌细胞. 未经TNF-α作用的胃癌细胞作为对照. 采用ELISA法检测胃癌细胞培养上清液中MMP-2和MMP-9蛋白的含量. 采用RT-PCR检测胃癌细胞MMP-2 mRNA和MMP-9 mRNA的表达状况. 采用Western blot检测胃癌细胞内MMP-2蛋白和MMP-9蛋白含量.
结果: 不同浓度(1, 10和100 mg/L)的TNF-α作用胃癌细胞后, 胃癌细胞上清液中MMP-2的含量分别为8.24±1.36, 23.65±3.45和14.83±2.54 ng/L, 均显著高于对照组(1.56±0.23 ng/L, P<0.01); MMP-9的含量分别为12.47±2.66, 34.12±6.78和23.31±4.45 ng/L, 亦均明显高于对照组(5.25±0.89 ng/L, P<0.01), 其中以10 mg/L TNF-α作用时升高最为显著. TNF-α作用胃癌细胞16 h后, 上清液中MMP-2和MMP-9含量达到峰值, 分别为23.65±3.45和34.12±6.78 ng/L. 胃癌细胞MMP-2 mRNA和MMP-9 mRNA表达明显增强, 胃癌细胞内MMP-2和MMP-9蛋白表达亦明显增多.
结论: TNF-α可上调胃癌细胞MMP-2和MMP-9表达.
引文著录: 王峻, 郭德玉. TNF-α对胃癌细胞MMP-2和MMP-9表达的诱导作用. 世界华人消化杂志 2007; 15(11): 1208-1212
Revised: January 5, 2007
Accepted: January 27, 2007
Published online: April 18, 2007
AIM: To investigate the inductive effect of tumor necrosis factor-α (TNF-α) on the expression of matrix metalloproteinase-2 (MMP-2) and matrix metalloproteinase-9 (MMP-9) in gastric cancer cells.
METHODS: Gastric cancer cells were stimulated by exogenous TNF-α and the cells without stimulation served as controls. The contents of MMP-2 and MMP-9 in the supernatant fluid of gastric cancer cells were measured by enzyme-linked immunosorbnent assay (ELISA). The expression of MMP-2 and MMP-9 mRNA were detected by reverse transcription-polymerase chain reaction (RT-PCR). Western blot was used to detect the expression of MMP-2 and MMP-9 protein in gastric cancer cells.
RESULTS: Following stimulation with 1, 10 and 100 mg/L TNF-α, the contents of MMP-2 (8.24 ± 1.36, 23.65 ± 3.45, 14.83 ± 2.54 ng/L) and MMP-9 (12.47 ± 2.66, 34.12 ± 6.78, 23.31 ± 4.45 ng/L) in the supernatant fluid of gastric cancer cells were increased significantly as compared with those in the control group (MMP-2: 1.56 ± 0.23 ng/L; 5.25 ± 0.89 ng/L; all P < 0.01). After 16 h of stimulation with TNF-α, the contents of MMP-2 and MMP-9 in the supernatant fluid reached the peak values, which were 23.65 ± 3.45 and 34.12 ± 6.78 ng/L, respectively. MMP-2 and MMP-9 expression were up-regulated both at mRNA and protein level.
CONCLUSION: TNF-α can up-regulate the expression of MMP-2 and MMP-9 in gastric cancer cells.
- Citation: Wang J, Guo DY. Induction of matrix metalloproteinase-2 and matrix metalloproteinase-9 expression in gastric cancer cells by tumor necrosis factor-α. Shijie Huaren Xiaohua Zazhi 2007; 15(11): 1208-1212
- URL: https://www.wjgnet.com/1009-3079/full/v15/i11/1208.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v15.i11.1208
胃癌作为人类最常见的肿瘤之一, 常于诊断前侵入肌层并发生转移[1], 因此患者的5年生存率较低, 具有较高的死亡率. 基质金属蛋白酶(MMPs)家族成员中的MMP-9和MMP-2能有效的分解基底膜的主要成分IV型胶原蛋白, 两者的过度表达常引起胃癌的转移侵袭能力增强[2-3]. 众多的研究显示, TNF-α参与了部分胃癌的发生、发展过程[4-5], 与胃癌的的侵袭转移能力具有相关性. 我们采用外源性TNF-α刺激胃癌细胞, 同时对MMP-2和MMP-9表达情况进行检测, 分析TNF-α对胃癌细胞MMP-2和MMP-9表达的影响, 以探讨TNF-α在胃癌进展过程中的作用.
人类胃癌细胞系SGC-7901由中国医科大学肿瘤研究所惠赠. 细胞培养于RPMI1640培养基中, 其中含有100 mL/L肽牛血清, 青霉素 (100 IU/mL)及链霉素(100 mg/L). TNF-α购买于美国Sigma公司. MMP-2和MMP-9 ELISA试剂盒购自英国Biotrak Amersham公司. RNA PCR3.0试剂盒及TRIzol试剂均购自于TaKaRa公司. 所有检测均重复4次.
1.2.1 RNA提取: 采用TRIzol法, 具体方法按其说明进行.
1.2.2 逆转录聚合酶链反应(RT-PCR): 取1 mg SFRP1 RNA, 采用随机9引物法和AMV逆转录酶合成cDNA. 循环条件为: 30 ℃, 10 min; 42 ℃, 25 min; 99 ℃, 5 min; 5 ℃, 5 min, 共1个循环. MMP-2的PCR引物序列分别为: 上游5'-GTGCCAAGGTGGAAATCAGAG-3'; 下游5'-AAGGTTGAAGGAAACGAGCGA-3'. 循环条件为: 94 ℃, 2 min, 1个循环. 94 ℃, 30 s; 60 ℃, 30 s; 72 ℃, 2 min, 共30个循环. MMP-9的PCR引物序列分别为上游5'-GTCTTCCCCTTCGTCTTCCT-3', 下游5'-ACCCCACTTCTTGTCAGCGT-3'. 循环条件为: 94 ℃, 2 min, 1个循环. 94 ℃, 30 s; 60 ℃, 30 s; 72 ℃, 2 min, 共30个循环.
1.2.3 ELISA测定培养胃癌细胞上清中MMP-2和MMP-9蛋白的含量: 具体操作按其说明书进行.
1.2.4 蛋白印迹(Western blot): 取蛋白20 mg, 100 g/L SDS-PAGE凝胶电泳, 常规转膜, 加抗MMP-2, MMP-9的多克隆抗体(1:1000), 4 ℃过夜后, 加辣根过氧化物酶标记兔抗羊Ⅱ抗(1:1000). 放射自显影后凝胶成像分析系统分析.
统计学处理 用SPSS11.0统计软件进行方差分析和t检验.
分别采用1, 10和100 mg/L TNF-α作用胃癌细胞, 未经TNF-α作用的细胞作为对照. 16 h后, 采用ELISA法检测胃癌细胞上清液中MMP-2和MMP-9的含量. 结果显示, 在不同浓度的TNF-α作用下, 胃癌细胞上清液中MMP-2和MMP-9含量均显著增加(P<0.01), 其中以10 mg/L TNF-α作用胃癌细胞时, MMP-2和MMP-9增加最为显著(表1).
以10 mg/L的TNF-α作用胃癌细胞, 在1、4、16和24 h后分别收集上清. 采用ELISA法对上清液中MMP-2和MMP-9含量进行检测. 结果显示, TNF-α作用后, 随着时间的推移, MMP-2和MMP-9的含量增加显著(P<0.01), 而峰值出现在16 h后(表2).
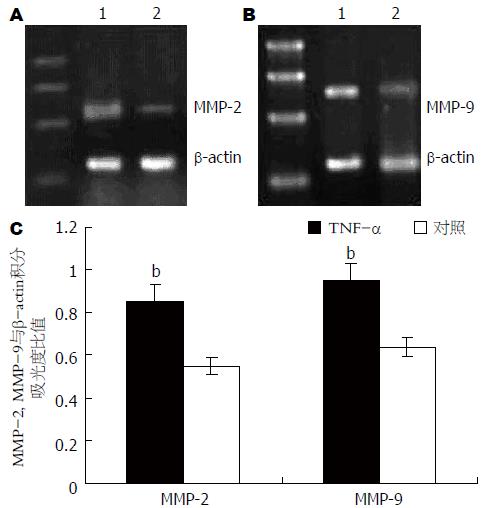
采用浓度为10 mg/L的TNF-α作用于胃癌细胞, 于16 h后收集细胞并采用RT-PCR对MMP-2 mRNA和MMP-9 mRNA表达进行检测. 同时, 对未经TNF-α作用的胃癌细胞MMP-2 mRNA和MMP-9 mRNA进行检测, 以作为对照. 运用凝胶成像分析系统进行半定量分析, 以MMP-2, MMP-9与β-actin的条带积分吸光度比值表示MMP-2 mRNA和MMP-9 mRNA的表达水平. 结果显示, 经TNF-α作用后, 胃癌细胞MMP-2 mRNA和MMP-9 mRNA表达均明显增强(图1).
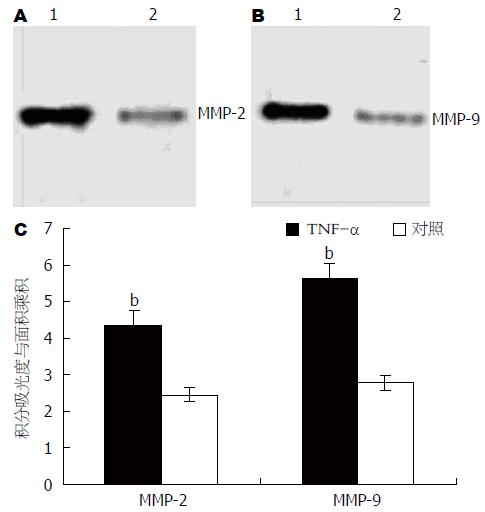
采用浓度为10 mg/L的TNF-α作用于胃癌细胞, 于16 h后收集细胞并采用Western blot对MMP-2蛋白和MMP-9蛋白表达进行检测. 同时, 对未经TNF-α作用的胃癌细胞MMP-2蛋白和MMP-9蛋白进行检测, 以作为对照. 凝胶成像分析系统对条带进行积分吸光度分析. 用积分吸光度与条带面积的乘积对蛋白表达进行半定量分析. 结果显示, 经TNF-α作用后, 胃癌细胞MMP-2蛋白和MMP-9蛋白表达均明显增强(图2).
目前, 越来越多的证据显示炎症反应是肿瘤发生过程中的一个重要因素[6-7]. 作为Ⅰ类致癌原, 幽门螺杆菌感染导致的慢性炎症则被认为是胃癌发病的重要原因之一. 幽门螺杆菌常诱导胃内黏膜TNF-α表达增多[8-10], 后者可有效抑制胃酸的分泌[11], 进一步促进幽门螺杆菌生长, 导致炎症的发生. 尽管TNF-α被认为能够导致肿瘤发生溶血性坏死, 然而更多的证据却显示, TNF-α在促进肿瘤发生、发展的过程中起到重要作用. 例如, 在动物实验中, 肿瘤诱导剂TPA等可诱导多个器官出现TNF-α基因表达增加[12]. TNF-α本身也是一个肿瘤诱导剂, 他能诱导BALB/3T3细胞出现恶性转化[13]. 此外亦有实验证明, TNF-α缺乏小鼠可抵抗TPA等诱导的小鼠皮肤肿瘤出现[14]. 这些证据均表明, TNF-α是促进胃癌发生、发展的一个重要的细胞因子.
作为一种常见的恶性肿瘤, 胃癌致死率在癌症中占第2位. 其较高的致死率常与其较早发生侵袭转移有关. 研究显示, 基质金属蛋白酶家族中部分成员与多种恶性肿瘤的侵袭性和转移性增强有关[15-23]. 金属基质蛋白酶能够降解基底膜和细胞外基质中的主要成分胶原, 从而使肿瘤细胞可以浸润到周围组织并向远处转移. 因此胃癌常于诊断前侵入肌层并发生转移, 患者的5年生存率较低, 具有较高的死亡率. 有证据显示, TNF-α可诱导多种细胞表达MMPs, 参与了多种疾病的发病过程. 例如, TNF-α能诱导肺上皮细胞[24]MMP-9表达, 参与了肺结核的发病过程. 周围神经损伤, TNF-α亦可调节施旺细胞表达MMP-9. TNF-α也可促进支气管平滑肌细胞和小肠上皮细胞[25]表达MMP-9. 肺上皮细胞、血管内皮细胞[26]及成纤维细胞[27-28]在TNF-α作用下MMP-2表达亦增加. 有证据显示, TNF-α对MMPs的诱导作用主要是通过MAPK信号系统介导的NF-κB激活, 例如TNF-α即可通过该通路上调血管平滑肌细胞MMP-9的表达[29-30].
本实验结果显示, TNF-α可上调胃癌上皮细胞MMP-2和MMP-9的表达, 且在10 mg/L时MMP-2和MMP-9表达达到峰值. 实验结果亦显示, 在TNF-α作用后16 h左右, MMP-2和MMP-9表达最多. 由于在胃癌患者中, TNF-α高表达常引起肿瘤早期出现侵袭和转移, 其机制亦可能与MMPs表达上调有关. 我们实验的结果进一步证实, TNF-α参与了胃癌的进展过程. 关于TNF-α对胃癌细胞MMP-2和MMP-9的诱导作用的具体机制则有待于进一步研究.
尽管TNF-α被认为能够导致肿瘤发生溶血性坏死, 然而更多的证据却显示TNF-α在促进肿瘤发生、发展的过程中起到重要作用. 另有研究显示, 基质金属蛋白酶家族中部分成员与多种恶性肿瘤的侵袭性和转移性增强有关.
本文首次分析了TNF-α对胃癌细胞MMP-2和MMP-9表达的影响. 结果发现, TNF-α能够上调MMP-2和MMP-9表达, 提示TNF-α在胃癌发生、发展过程中具有重要作用.
本文研究了TNF-α对胃癌细胞MMP-2和MMP-9表达的诱导作用, 方法尚可, 结论可信, 有一定实际价值.
编辑: 张焕兰 电编:张敏
| 2. | Wu ZY, Li JH, Zhan WH, He YL. Lymph node micrometastasis and its correlation with MMP-2 expression in gastric carcinoma. World J Gastroenterol. 2006;12:2941-2944. [PubMed] [DOI] |
| 3. | Dicken BJ, Graham K, Hamilton SM, Andrews S, Lai R, Listgarten J, Jhangri GS, Saunders LD, Damaraju S, Cass C. Lymphovascular invasion is associated with poor survival in gastric cancer: an application of gene-expression and tissue array techniques. Ann Surg. 2006;243:64-73. [PubMed] [DOI] |
| 4. | Macrì A, Versaci A, Loddo S, Scuderi G, Travagliante M, Trimarchi G, Teti D, Famulari C. Serum levels of interleukin 1beta, interleukin 8 and tumour necrosis factor alpha as markers of gastric cancer. Biomarkers. 2006;11:184-193. [PubMed] [DOI] |
| 5. | Fei BY, Xia B, Deng CS, Xia XQ, Xie M, Crusius JB, Pena AS. Association of tumor necrosis factor genetic polymorphism with chronic atrophic gastritis and gastric adenocarcinoma in Chinese Han population. World J Gastroenterol. 2004;10:1256-1261. [PubMed] |
| 7. | Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7:211-217. [PubMed] [DOI] |
| 8. | Peek RM, Miller GG, Tham KT, Perez-Perez GI, Zhao X, Atherton JC, Blaser MJ. Heightened inflammatory response and cytokine expression in vivo to cagA+ Helicobacter pylori strains. Lab Invest. 1995;73:760-770. [PubMed] |
| 9. | Crabtree JE, Shallcross TM, Heatley RV, Wyatt JI. Mucosal tumour necrosis factor alpha and interleukin-6 in patients with Helicobacter pylori associated gastritis. Gut. 1991;32:1473-1477. [PubMed] [DOI] |
| 10. | Hwang IR, Kodama T, Kikuchi S, Sakai K, Peterson LE, Graham DY, Yamaoka Y. Effect of interleukin 1 polymorphisms on gastric mucosal interleukin 1beta production in Helicobacter pylori infection. Gastroenterology. 2002;123:1793-1803. [PubMed] [DOI] |
| 11. | Beales IL, Calam J. Interleukin 1 beta and tumour necrosis factor alpha inhibit acid secretion in cultured rabbit parietal cells by multiple pathways. Gut. 1998;42:227-234. [PubMed] [DOI] |
| 12. | Fujiki H, Suganuma M. Tumor promotion by inhibitors of protein phosphatases 1 and 2A: the okadaic acid class of compounds. Adv Cancer Res. 1993;61:143-194. [PubMed] [DOI] |
| 13. | Komori A, Yatsunami J, Suganuma M, Okabe S, Abe S, Sakai A, Sasaki K, Fujiki H. Tumor necrosis factor acts as a tumor promoter in BALB/3T3 cell transformation. Cancer Res. 1993;53:1982-1985. [PubMed] |
| 14. | Moore RJ, Owens DM, Stamp G, Arnott C, Burke F, East N, Holdsworth H, Turner L, Rollins B, Pasparakis M. Mice deficient in tumor necrosis factor-alpha are resistant to skin carcinogenesis. Nat Med. 1999;5:828-831. [PubMed] [DOI] |
| 15. | Kim SH, Choi HY, Lee J, Son DS, Lee HS, Song IS, Lim YS, Hong YS, Kim J, Choi YS. Elevated activities of MMP-2 in the non-tumorous lung tissues of curatively resected stage I NSCLC patients are associated with tumor recurrence and a poor survival. J Surg Oncol. 2007;95:337-346. [PubMed] [DOI] |
| 16. | Hu XX, Li L, Li DR, Zhang W, Tang BJ. Inhibitory effects of antisense MMP-9 oligodeoxynucleotides on invasiveness and adherence of ovarian cancer cells. Zhonghua Zhongliu Zazhi. 2006;28:662-665. [PubMed] |
| 17. | Kang HG, Kim HS, Kim KJ, Oh JH, Lee MR, Seol SM, Han I. RECK expression in osteosarcoma: correlation with matrix metalloproteinases activation and tumor invasiveness. J Orthop Res. 2007;25:696-702. [PubMed] [DOI] |
| 18. | Ip YC, Cheung ST, Fan ST. Atypical localization of membrane type 1-matrix metalloproteinase in the nucleus is associated with aggressive features of hepatocellular carcinoma. Mol Carcinog. 2007;46:225-230. [PubMed] [DOI] |
| 19. | Ko HM, Kang JH, Jung B, Kim HA, Park SJ, Kim KJ, Kang YR, Lee HK, Im SY. Critical role for matrix metalloproteinase-9 in platelet-activating factor-induced experimental tumor metastasis. Int J Cancer. 2007;120:1277-1283. [PubMed] [DOI] |
| 20. | Ou DP, Tao YM, Tang FQ, Yang LY. The hepatitis B virus X protein promotes hepatocellular carcinoma metastasis by upregulation of matrix metalloproteinases. Int J Cancer. 2007;120:1208-1214. [PubMed] [DOI] |
| 21. | Zhang Y, Wang C, Mizukami H, Itoh H, Kusama M, Ozawa K, Jinbu Y. Increased expression and activation of matrix metalloproteinase-2 (MMP-2) in O-1N: hamster oral squamous cell carcinoma with high potential lymph node metastasis. J Exp Clin Cancer Res. 2006;25:417-423. [PubMed] |
| 22. | Sahin A, Kiratli H, Tezel GG, Soylemezoglu F, Bilgic S. Expression of vascular endothelial growth factor a, matrix metalloproteinase 9 and extravascular matrix patterns in iris and ciliary body melanomas. Ophthalmic Res. 2007;39:40-44. [PubMed] [DOI] |
| 23. | Petrella BL, Brinckerhoff CE. Tumor cell invasion of von Hippel Lindau renal cell carcinoma cells is mediated by membrane type-1 matrix metalloproteinase. Mol Cancer. 2006;5:66. [PubMed] [DOI] |
| 24. | Elkington PT, Friedland JS. Matrix metalloproteinases in destructive pulmonary pathology. Thorax. 2006;61:259-266. [PubMed] [DOI] |
| 25. | Santana A, Medina C, Paz-Cabrera MC, Díaz-Gonzalez F, Farré E, Salas A, Radomski MW, Quintero E. Attenuation of dextran sodium sulphate induced colitis in matrix metalloproteinase-9 deficient mice. World J Gastroenterol. 2006;12:6464-6472. [PubMed] [DOI] |
| 26. | Lin R, Liu JT, Gan WJ, Wang WR, Han CJ, Liu Y, Fang ZY. Fenofibrate inhibits tumor necrosis factor-alpha-induced expression of CD40 and matrix metalloproteinase in human vascular endothelial cells. Nanfang Yike Daxue Xuebao. 2006;26:1383-1387. [PubMed] |
| 27. | Wisithphrom K, Windsor LJ. The effects of tumor necrosis factor-alpha, interleukin-1beta, interleukin-6, and transforming growth factor-beta1 on pulp fibroblast mediated collagen degradation. J Endod. 2006;32:853-861. [PubMed] [DOI] |
| 28. | Braundmeier AG, Nowak RA. Cytokines regulate matrix metalloproteinases in human uterine endometrial fibroblast cells through a mechanism that does not involve increases in extracellular matrix metalloproteinase inducer. Am J Reprod Immunol. 2006;56:201-214. [PubMed] [DOI] |
| 29. | Lee SO, Jeong YJ, Yu MH, Lee JW, Hwangbo MH, Kim CH, Lee IS. Wogonin suppresses TNF-alpha-induced MMP-9 expression by blocking the NF-kappaB activation via MAPK signaling pathways in human aortic smooth muscle cells. Biochem Biophys Res Commun. 2006;351:118-125. [PubMed] [DOI] |
| 30. | Suh SJ, Jin UH, Kim SH, Chang HW, Son JK, Lee SH, Son KH, Kim CH. Ochnaflavone inhibits TNF-alpha-induced human VSMC proliferation via regulation of cell cycle, ERK1/2, and MMP-9. J Cell Biochem. 2006;99:1298-1307. [PubMed] [DOI] |