修回日期: 2006-03-15
接受日期: 2006-03-16
在线出版日期: 2006-03-18
目的: 观察肝细胞生长因子(hepatocyte growth factor, HGF)作用下, 脐血干细胞在体内向肝细胞分化的情况.
方法: HGF的裸DNA质粒通过尾静脉快速注入6-8周龄的非肥胖糖尿病/重症联合免疫缺陷(NOD/SCID)小鼠体内, 酶联免疫吸附法(ELISA)检测外周血中HGF水平. 收集足月妊娠产妇的脐血, 磁式分选法选出CD34+造血干细胞(hematopoietic stem cell, HSC). 20 μL四氯化碳(carbon tetrachloride, CCl4)腹腔注射建立NOD/SCID小鼠急性肝损伤模型, 分别输注CD34+HSC和/或HGF质粒, 观察其对死亡率、肝功能恢复的影响, 并检测小鼠肝组织内的人源性细胞.
结果: 快速注入HGF质粒后, 体内HGF水平明显上升. 实验各组存活率及肝功能恢复情况无明显差异. 病理切片显示, 联合应用HGF+HSC的实验组, 其肝组织损伤程度最轻, 单独应用HGF或HSC的两个实验组结果类似, 对照组最重. 在两组注射HSC的小鼠肝组织中, 均可检测到人源性的、分泌白蛋白的肝样细胞, 且联合应用HGF的实验组中, 此种细胞更多, 分布更广.
结论: HGF可以促进脐血干细胞向肝细胞分化, 并发挥肝细胞功能.
引文著录: 范烨, 王学浩, 张峰, 李相成, 王科, 钱晓峰. HGF质粒体内表达诱导脐血干细胞向肝系细胞分化. 世界华人消化杂志 2006; 14(8): 767-771
Revised: March 15, 2006
Accepted: March 16, 2006
Published online: March 18, 2006
AIM: To observe the hepatocyte growth factor-induced differentiation of CD34+ hematopoietic stem cells (HSC) from umbilical cord blood into hepatocyte-like cells.
METHODS: Systemic administration of naked plasmid containing HGF cDNA driven under cytomegalovirus promoter (pCMV-HGF) were injected rapidly via the tail vein of the NOD/SCID mice, and the level of HGF protein in the peripheral blood was detected by enzyme-linked immunosorbent assay. CD34+ human hematopoietic stem cells were isolated from umbilical cord blood by magnetic cell sorting method. 20 μL CCl4 was administered into the mice to establish the model of acute liver damage and hepatocyte proliferation. pCMV-HGF injection and/or CD34+ human hematopoietic stem cells transplantation were performed on the model mice. Then the mortality of the mice and liver function recovery status were observed. Human specific mRNA and protein were also detected in the mice by reverse transcription polymerase chain reaction and immunohistochemistry, respectively (RT-PCR).
RESULTS: A remarkable enhancement of human HGF protein level was observed in the peripheral blood of the mice. The mortality and status of liver function were not significantly different between each experiment group. Path-ological examination showed that the mice received combined treatment HGF and HSC had the lightest liver injury, while the liver injury was not markedly different between the mice received HGF and HSC alone. Human albumin mRNA and protein were all expressed in the liver tissues underwent HSC transplantation with or without HGF, while in the mice with HGF injection, there were much more hepatocyte-like cells.
CONCLUSION: Stem cells from umbilical cord blood can differentiate into hepatocyte-like cells, and HGF can promote this process.
- Citation: Fan Y, Wang XH, Zhang F, Li XC, Wang K, Qian XF. In vivo expression of hepatocyte growth factor promotes differentiation of stem cells from umbilical cord blood into hepatocyte-like cells. Shijie Huaren Xiaohua Zazhi 2006; 14(8): 767-771
- URL: https://www.wjgnet.com/1009-3079/full/v14/i8/767.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v14.i8.767
近年来, 随着干细胞可塑性研究的不断深入, 同样发现其在体内外均可向肝脏细胞分化, 但最佳诱导条件及分化后细胞是否具有成熟功能尚未明确. 本实验中, 我们采用HGF质粒尾静脉快速注射得到小鼠体内HGF的高表达, 并进一步来诱导外源性造血干细胞向肝脏细胞的分化, 观察其是否可以修复受损的肝脏.
非肥胖糖尿病/重症联合免疫缺陷(NOD/SCID)小鼠, 6-8周龄, 均由南京大学模式动物研究所提供. HGF ELISA检测试剂盒为Biosource公司产品, Ficoll-Paque(密度1.077)及EasySepTN免疫磁珠阳性选择分离系统购自加拿大Stemcell公司; 人肝细胞单克隆一抗购自日本Dako公司; 人ALB多克隆一抗购自Rockland公司.
1.2.1 HGF基因体内转染: pCMV-HGF质粒由杨俊伟教授惠赠, 通过尾静脉一次性快速注入1.6 mL含有20 μg此种质粒的溶液[1], 注射后4, 8, 12, 24, 72, 144 h分别采血, ELISA法检测外周血中HGF水平.
1.2.2 人脐血CD34+造血干细胞的分离纯化: 选择我院足月妊娠产妇, 产前检查HBsAg和抗-HCV均阴性, 收集其脐血. 密度梯度离心分离单个核细胞, 再通过免疫磁式分选法收集CD34+的HSC. 纯化得到的干细胞直接用于细胞移植, 或加入冻存液后置于液氮中备用.
1.2.3 干细胞移植及体内诱导分化: CCl4 20 μL, 用矿物质油稀释至100 μL, 腹腔内注射, 建立小鼠肝损伤模型, 24 h后尾静脉注射pCMV-HGF(20 μg, 1.6 mL)和/或48 h后尾静脉注射CD34+HSC(细胞数量: 5-10×105/鼠). 实验分四组:Ⅰ组: 联合应用HGF+HSC(n = 8); Ⅱ组: 单独应用pCMV-HGF(n = 8); Ⅲ组: 单独应用CD34+HSC(n = 8); Ⅳ组: 对照组, CCl4建立小鼠肝损伤模型(n = 6). 观察建模14 d后各组的存活率; 建模后第3, 5, 7, 10, 14 d小鼠眼眶取血, 测定血清中ALT、AST水平, 评价各组肝功能恢复状况.
1.2.4 处死后处理: 4 wk后处死所有小鼠: (1)制作肝组织石蜡切片, HE染色观察; (2)RT-PCR: 引物设计[2], 白蛋白, 5'-TTGGAAAAATCCCACT-GCAT, 5'-CTCCAAGCTGCTCAAAAAGC; GAPDH, 5'-TGAAGGTCGGTGTGAACGGATTTGGC, 5'-TGTTGGGGGCCGAGTTGGGATA. Trizol试剂提取细胞总RNA, 扩增条件: 94℃ 2 min, 94℃ 30 s, 55℃ 30 s, 72℃ 50 s, 30个循环, 72℃ 4 min. 将扩增的RT-PCR产物电泳成像; (3)免疫组化: 含40 g/L多聚甲醛的PBS中室温下固定, 参照试剂盒说明逐步操作, 加入人ALB多抗、人肝细胞单抗等一系列步骤, 最后DAB显色, 封片后光镜下观察, 阳性染色细胞为棕黄色或棕褐色.
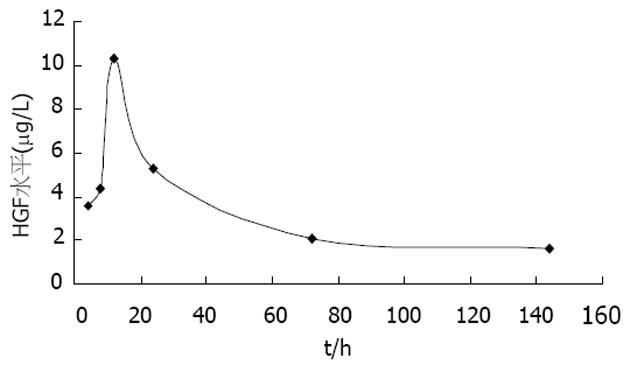
如图1所示, 快速注射后4 h即可见到HGF水平明显上升, 12 h达到顶峰, 然后逐渐下降, 但6 d后仍可测到较高水平的HGF.
实验各组14 d存活率相近, 肝功能恢复无明显差异, Ⅰ组第5, 7 d ALT、AST水平较其余各组低, 但无统计学意义(表1, 2).
| 实验组 | 实验操作 | 存活率 |
| Ⅰ组 | HGF+HSC | 7/8(87.5%) |
| Ⅱ组 | HGF | 8/8(100%) |
| Ⅲ组 | HSC | 7/8(87.5%) |
| Ⅳ组 | 对照组 | 4/6(66.7%) |
| 时间(d) | Ⅰ组 | Ⅱ组 | Ⅲ组 | Ⅳ组 | |
| 3 | ALT | 2210.4±393.4 | 1980.4±271.7 | 2083.8±478.4 | 2207.1±448.4 |
| AST | 7008.0±1281.9 | 7571.5±1493.6 | 6639.7±1155.2 | 7336.5±1445.3 | |
| 5 | ALT | 1563.6±471.7 | 1778.7±541.8 | 1933.7±525.1 | 1833.7±410.1 |
| AST | 5237.7±1093.6 | 5877.8±988.5 | 6184.6±1105.2 | 6609.7±1308.6 | |
| 7 | ALT | 1280.3±310.1 | 1600.3±256.7 | 1370.3±370.1 | 1472.0±526.8 |
| AST | 4382.5±543.4 | 5001.0±1065.2 | 4901.0±978.5 | 5704.5±785.2 | |
| 10 | ALT | 1136.9±360.1 | 1246.9±220.0 | 1166.9±310.1 | 1271.9±471.8 |
| AST | 3949.1±903.5 | 3654.1±866.8 | 4724.3±398.4 | 3999.1±615.1 | |
| 14 | ALT | 1068.5±446.8 | 1158.6±385.1 | 1048.5±423.4 | 1066.9±295.1 |
| AST | 2805.6±793.5 | 2623.9±815.2 | 3235.6±805.2 | 3447.4±893.5 |
建模后4 wk, 各组小鼠肝脏仍有不同程度的损伤性改变, Ⅰ组最轻, 肝索完整, 肝细胞无明显肿胀, Ⅳ组最重, 组织结构紊乱, 细胞浊肿, Ⅱ组、Ⅲ组介于前两组之间.
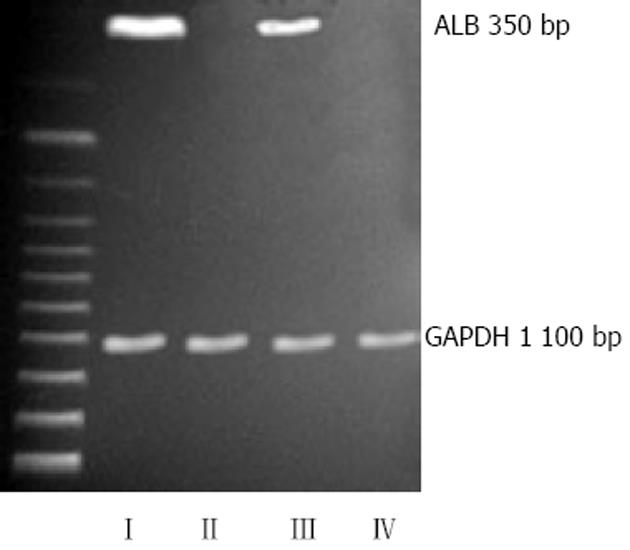
如图2所示, Ⅰ、Ⅲ组中均可扩增出人白蛋白的mRNA(350 bp), 且HSC与HGF联用的Ⅰ组表达量更高, 其余两组则为阴性.
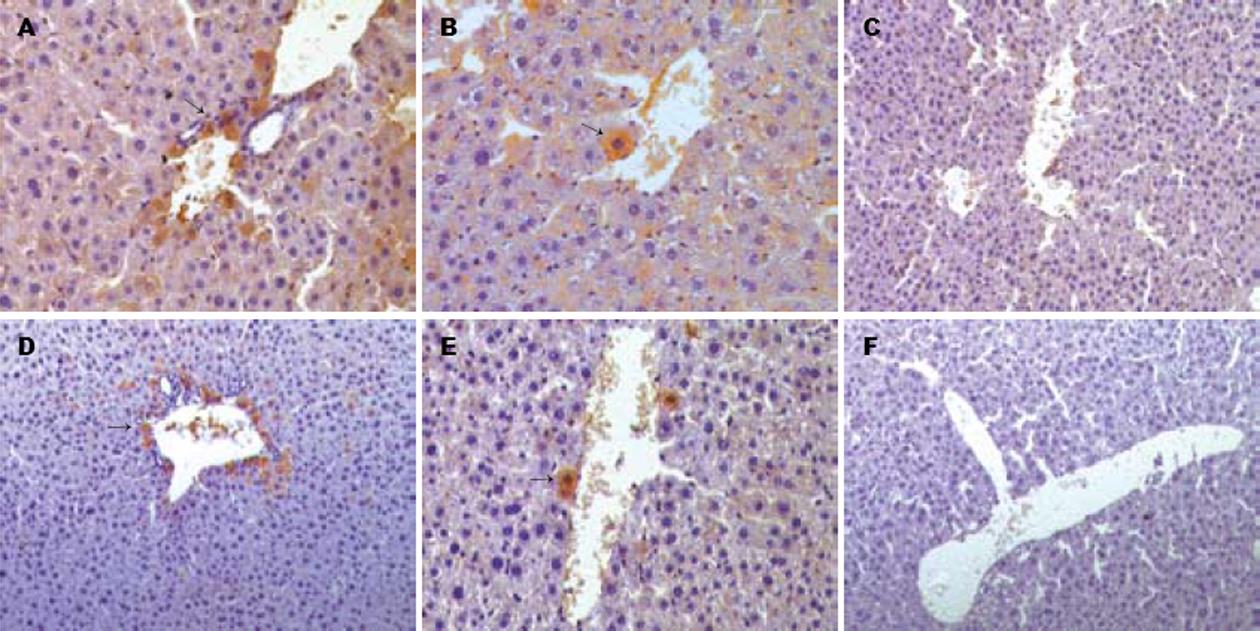
造血干细胞移植的两组(Ⅰ组、Ⅲ组)中, 均可以观察到人源性的肝脏实质细胞, 抗人ALB抗体(+), 抗人肝细胞抗体(+), 而另两组为阴性. 肝脏内阳性细胞分布以汇管区为主. 与Ⅲ组比较, Ⅰ组中阳性细胞所占比例更高, 分布更广, 且整合入肝板中(图3).
急性肝功能衰竭是一种死亡率很高的疾病, 起病急、进展快, 预后极差, 其治疗已由传统的保肝解毒、支持治疗向肝细胞移植、生物人工肝及肝移植方向发展[3-5]. 肝细胞移植具有操作简单、损伤小、疗效确实等优点, 其主要机制包括提供暂时的肝功能支持, 以及通过产生HGF促进残存肝细胞的再生和功能恢复[6-7]. 但由于细胞来源的匮乏, 限制了其在临床上的广泛应用, 而异种肝细胞移植不可避免的免疫排斥反应又会严重影响治疗效果.
近年来发现, 骨髓或脐带血中的干细胞可以向肝系细胞分化[8-12], 进一步研究[2,13-15]表明, CD34+干细胞亚群具有较好的定向分化潜能, 有望成为生物人工肝或肝细胞移植新的供体细胞来源.
在免疫缺陷小鼠急性肝功能损伤模型中, 将人干细胞输入体内后可自发迁移入肝脏, 分化成为肝样细胞, 分泌白蛋白, 或者与宿主肝细胞融合, 而将干细胞体外培养时, 添加一定剂量的HGF及其他生长因子, 或者与肝细胞共孵育均同样可以诱导其向肝样细胞分化[16-18], 但Lian et al[19]认为体外诱导分化的干细胞无法完全转化成为成熟的肝细胞, 此分化过程的发生有待于进一步确认. 而在体内实验时, 由于更接近于实际, 在早期补充外源性HGF, 理论上可以进一步促进干细胞的分化进程. 将干细胞注入肝损伤小鼠体内, 发现可以减轻损伤程度, 降低死亡率[20-21]. 我们考虑通过尾静脉输注造血干细胞并在体内诱导其向肝脏细胞分化, 从而改善肝功能状况.
Yang et al[1]的研究显示, 将HGF质粒通过尾静脉快速输注入小鼠体内, 在早期HGF水平即可有较大的提高. 我们将此实验在免疫缺陷小鼠上重复, 得到了类似结果, HGF水平升高可持续将近1 wk. 通过此种转基因方式, 既得到了HGF的体内高表达, 同时避免了使用载体所带来的各种副作用, 包括对肝脏的毒性. 由于这种HGF是由自身细胞分泌, 而非外源性导入, 从而可以保证其活性并减少了输注的风险. 使用体内这种高水平的HGF来诱导干细胞分化, 较之体外实验更接近于实际, 且理论上分化得到的肝细胞功能应该更成熟. 采用CCl4腹腔注射建立急性肝损伤模型, 对肝脏微血管影响较小, 有利于干细胞向肝脏内的迁移、分化[13]. 本研究结果表明, 这种方法可以促进干细胞进一步向肝样细胞的分化, 其细胞数量及分布范围均有明显增加. 但现有数据显示各组存活率及肝功能恢复无明显差异, 虽然病理检查提示损伤程度可以得到一定程度的改善, 但无法证实分化而来的肝样细胞可以完全代偿成熟肝细胞的作用, 其生物学功能并不明确, 能否修复损伤的肝脏尚有待于进一步研究.
干细胞的生物学特性决定了其具有巨大的应用前景, 尤其在移植领域, 不但可有效解决供体细胞来源问题, 而且可通过自体干细胞移植或核移植技术的预处理来避免免疫排斥反应的发生, 并通过对移植干细胞的基因修饰来纠正患者的先天性缺陷
近来发现来源于骨髓或脐血的干细胞可在体外或体内向肝脏细胞分化, 本文拟在HGF质粒转染得到体内高表达的基础上, 诱导脐血CD+造血干细胞向肝脏细胞分化, 并修复受损肝脏.
干细胞向肝脏细胞分化可能存在两种机制: 直接分化或与宿主原有肝细胞融合, 何种干细胞亚群更易向肝脏细胞分化及体内外最佳诱导条件尚有争论, 分化得到的肝细胞是否具有成熟的功能亦未明确.
本研究将HGF质粒通过尾静脉快速输注入小鼠体内, 在早期HGF水平即可有较大的提高, 且可持续将近1 wk. 既得到了HGF的体内高表达, 同时避免了使用载体所带来的各种副作用. 结果表明, 这种方法可以促进干细胞进一步向肝样细胞的分化, 其细胞数量及分布范围均有明显增加.
HGF质粒可大量扩增获得, 且高表达的HGF是由自身细胞分泌, 而非外源性导入, 从而可以保证其活性并减少输注的风险. 通过此种方法诱导干细胞分化, 更接近于体内实际, 所得到的细胞理论上应该更成熟.
本文通过裸DNA质粒尾静脉快速注入技术, 研究HGF体内表达与脐血干细胞的肝系分化, 发现快速注入HGF质粒后, 体内HGF水平明显上升. 在两组注射HSC的小鼠肝组织中, 均可检测到人源性的、分泌白蛋白的肝样细胞, 且联合应用HGF的实验组中, 此种细胞更多, 分布更广. 提示HGF可以促进脐血干细胞向肝细胞分化, 并发挥肝细胞功能. 文章设计合理, 结果可信, 具有一定的理论与实践意义.
电编: 张敏 编辑:张海宁
| 1. | Yang J, Chen S, Huang L, Michalopoulos GK, Liu Y. Sustained expression of naked plasmid DNA encoding hepatocyte growth factor in mice pro-motes liver and overall body growth. Hepatology. 2001;33:848-859. [PubMed] [DOI] |
| 2. | Kakinuma S, Tanaka Y, Chinzei R, Watanabe M, Shimizu-Saito K, Hara Y, Teramoto K, Arii S, Sato C, Takase K. Human umbili-cal cord blood as a source of transplantable hepatic progenitor cells. Stem Cells. 2003;21:217-227. [PubMed] [DOI] |
| 3. | Nyberg SL, Hay EJ, Ramin KD, Rosen CB. Successful pregnancy after porcine bioartificial liver treatment and liver transplantation for fulminant hepatic failure. Liver Transpl. 2002;8:169-170. [PubMed] [DOI] |
| 4. | Fox IJ. Transplantation into and inside the liver. Hepatology. 2002;36:249-251. [PubMed] [DOI] |
| 5. | Sarkis R, Honiger J, Chafai N, Baudrimont M, Sarkis K, Delelo R, Becquemont L, Benoist S, Balladur P, Capeau J. Semiautomatic macroencapsulation of fresh or cryopreserved por-cine hepatocytes maintain their ability for treatment of acute liver failure. Cell Transplant. 2001;10:601-607. [PubMed] |
| 6. | Strain AJ, Neuberger JM. A bioartificial liver--state of the art. Science. 2002;295:1005-1009. [PubMed] [DOI] |
| 7. | Boudjema K, Bachellier P, Wolf P, Tempe JD, Jaeck D. Auxiliary liver transplantation and bioartificial bridging procedures in treatment of acute liver failure. World J Surg. 2002;26:264-274. [PubMed] [DOI] |
| 8. | Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase N, Boggs SS, Greenberger JS, Goff JP. Bone marrow as a potential source of hepatic oval cells. Science. 1999;284:1168-1170. [PubMed] [DOI] |
| 9. | Theise ND, Badve S, Saxena R, Henegariu O, Sell S, Crawford JM, Krause DS. Derivation of hepatocytes from bone marrow cells in mice after radiation-induced myeloablation. Hepatology. 2000;31:235-240. [PubMed] [DOI] |
| 10. | Lagasse E, Connors H, Al-Dhalimy M, Reitsma M, Dohse M, Osborne L, Wang X, Finegold M, Weissman IL, Grompe M. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat Med. 2000;6:1229-1234. [PubMed] [DOI] |
| 11. | Lee KD, Kuo TK, Whang-Peng J, Chung YF, Lin CT, Chou SH, Chen JR, Chen YP, Lee OK. In vitro hepa-tic differentiation of human mesenchymal stem cells. Hepatology. 2004;40:1275-1284. [DOI] |
| 12. | Sharma AD, Cantz T, Richter R, Eckert K, Henschler R, Wilkens L, Jochheim-Richter A, Arseniev L, Ott M. Human cord blood stem cells generate human cytokeratin 18-negative hepatocyte-like cells in injured mouse liver. Am J Pathol. 2005;167:555-564. [PubMed] [DOI] |
| 13. | Wang X, Ge S, McNamara G, Hao QL, Crooks GM, Nolta JA. Albumin-expressing hepatocyte-like cells develop in the livers of immune-deficient mice that received transplants of highly purified human hematopoietic stem cells. Blood. 2003;101:4201-4208. [PubMed] [DOI] |
| 14. | Tanabe Y, Tajima F, Nakamura Y, Shibasaki E, Wakejima M, Shimomura T, Murai R, Murawaki Y, Hashiguchi K, Kanbe T. Analyses to clarify rich fractions in hepatic progenitor cells from human umbilical cord blood and cell fusion. Biochem Biophys Res Commun. 2004;324:711-718. [PubMed] [DOI] |
| 15. | Nava S, Westgren M, Jaksch M, Tibell A, Broome U, Ericzon BG, Sumitran-Holgersson S. Charac-terization of cells in the developing human liver. Differentiation. 2005;73:249-260. [PubMed] [DOI] |
| 16. | Quintana-Bustamante O, Alvarez-Barrientos A, Kofman AV, Fabregat I, Bueren JA, Theise ND, Segovia JC. Hematopoietic mobilization in mice increases the presence of bone marrow-derived hepatocytes via in vivo cell fusion. Hepatology. 2006;43:108-116. [PubMed] [DOI] |
| 17. | Takeda M, Yamamoto M, Isoda K, Higashiyama S, Hirose M, Ohgushi H, Kawase M, Yagi K. Availa-bility of bone marrow stromal cells in three-dimen-sional coculture with hepatocytes and transplan-tation into liver-damaged mice. J Biosci Bioeng. 2005;100:77-81. [PubMed] [DOI] |
| 18. | Lange C, Bassler P, Lioznov MV, Bruns H, Kluth D, Zander AR, Fiegel HC. Liver-specific gene expre-ssion in mesenchymal stem cells is induced by liver cells. World J Gastroenterol. 2005;11:4497-4504. [PubMed] [DOI] |
| 19. | Lian G, Wang C, Teng C, Zhang C, Du L, Zhong Q, Miao C, Ding M, Deng H. Failure of hepatocyte marker-expressing hematopoietic progenitor cells to efficiently convert into hepatocytes in vitro. Exp Hematol. 2006;34:348-358. [PubMed] [DOI] |
| 20. | Di Campli C, Piscaglia AC, Rutella S, Bonanno G, Vecchio FM, Zocco MA, Monego G, Michetti F, Mancuso S, Pola P. Improvement of mortality rate and decrease in histologic hepatic injury after human cord blood stem cell infusion in a murine model of hepatotoxicity. Transplant Proc. 2005;37:2707-2710. [PubMed] [DOI] |
| 21. | Liu ZC, Chang TM. Transplantation of bioencap-sulated bone marrow stem cells improves hepatic regeneration and survival of 90% hepatectomized rats: a preliminary report. Artif Cells Blood Substit Immobil Biotechnol. 2005;33:405-410. [PubMed] [DOI] |