修回日期: 2006-01-15
接受日期: 2006-01-25
在线出版日期: 2006-03-18
目的: 探讨胃癌组织Axin蛋白的表达与侵袭转移的相关性.
方法: 胃癌患者46例, 均行胃癌根治切除手术. 采集胃癌癌组织及远癌正常胃组织标本, 制备石蜡切片, 采用免疫组化(SABC)法检测Axin蛋白的表达, 研究其表达和分布特点与临床病理间的关系.
结果: 正常胃组织Axin蛋白在基底部细胞表达强, 表面成熟细胞中表达弱; 胃癌组织、远癌正常胃组织中均有Axin蛋白表达, 其阳性表达率分别为62.0%和91.3%, 差异有统计学意义(P<0.01); Axin蛋白的表达与胃癌临床病理分期(TNM Ⅰ,Ⅱ vs Ⅲ, Ⅳ: 78.6% vs 56.3%, P=0.035)、有无淋巴转移有关(无vs有: 85.7% vs 53.1%, P = 0.034), 与胃癌患者性别、年龄、肿瘤大小、生物学特征和是否侵及浆膜无关(P>0.05).
结论: Axin蛋白表达减弱与胃癌的发生以及肿瘤的临床进展和淋巴转移相关.
引文著录: 郑亚民, 李非, 董承伟, 伍晓汀. 胃癌组织 Axin 蛋白的表达与侵袭转移的相关性. 世界华人消化杂志 2006; 14(8): 763-766
Revised: January 15, 2006
Accepted: January 25, 2006
Published online: March 18, 2006
AIM: To investigate the relationship between Axin protein expression and genesis and metastasis of gastric carcinoma.
METHODS: A total of 46 patients with gastric carcinoma underwent surgical resection were included in this study. The expression of Axin protein was detected by immunohistochemical techniques (SABC) in paraffin-embedded samples prepared from gastric carcinoma tissues and normal stomach tissues far from the tumor. In addition, the positive rate of Axin expression was calculated and its corrrelation with the pathological characteristics of gastric carcinoma was analyzed.
RESULTS: In normal stomach tissues, Axin protein expression was strongly positive and intensified in basal cells. Axin was also expressed in tissues from gastric carcinoma and normal stomach mucosa far from the tumor with a positive rate of 62.0% and 91.3%, respectively. There was a significant difference between them (P < 0.01). Axin protein expression was significantly correlated with clinical TNM classification (TNM Ⅰ, Ⅱ vs Ⅲ, Ⅳ: 78.6% vs 56.3%, P = 0.035) and lymphatic metastasis (without vs with: 85.7% vs 53.1%, P = 0.034). The expression of Axin protein was not markedly correlated with the age and gender of patients, tumor size, biological features and serosa invasion (P > 0.05).
CONCLUSION: The expression of Axin protein is down-regulated in gastric carcinoma, which correlates with the genesis and metastasis of the disease.
- Citation: Zheng YM, Li F, Dong CW, Wu XT. Expression of Axin protein correlates with genesis and metastasis of gastric carcinoma. Shijie Huaren Xiaohua Zazhi 2006; 14(8): 763-766
- URL: https://www.wjgnet.com/1009-3079/full/v14/i8/763.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v14.i8.763
胃癌严重危害人类健康, 发病和侵袭转移机制的研究是当前的热点. 既往分子生物学研究发现, Wnt信号传导途径在调控细胞增殖、分化、运动性以及形态学等方面起着重要的作用[1-2]. Axin蛋白是Wnt信号途径的一个重要的负向调控因子[3-4], Axin基因被认为是抑癌基因. 现在发现多种肿瘤的发生和某些肿瘤的浸润转移与Axin基因异常有密切关系[5-9], 但对Axin蛋白表达异常与肿瘤发生、发展的关系研究较少, 我们探讨Axin蛋白的表达与胃癌发生及侵袭转移间的相关性.
2004-07/2005-03胃癌患者46例; 男性33例, 女性13例, 年龄30-75岁, 平均57.25±10.87岁. 全部施行胃癌根治切除术, 达到R0、D≥N; 获得术前胃镜或术中、术后病理证实. 术前未接受放化疗及生物治疗. 胃癌病理分期, Ⅰ期10例, Ⅱ期4例, Ⅲ期12例, Ⅳ期20例; 中、高分化腺癌19例, 低分化腺癌27例; 有淋巴转移者32例, 无淋巴转移者14例; 肿瘤直径大于5 cm患者16例, 小于5 cm患者30例; 侵及浆膜者33例, 未侵及浆膜者13例. 试剂: Axin(H-98)多克隆抗体, Santa Cruz Biotechnology, Inc USA; 山羊抗兔SABC免疫组化试剂盒(SA1022), 武汉博士德生物工程有限公司.
胃癌患者手术切除标本, 肉眼下观察记录肿瘤大小、性质、浸润深度. 立即切取直径0.4-1.5 cm大小(视肿块大小而定)胃癌组织, 在距离肿瘤6 cm以上远离癌肿处正常区切取1 cm直径大小正常胃组织(以上均经HE染色病理检查证实), 以40 g/L甲醛固定36-48 h, 常规石蜡包埋, 4 μm厚连续切片. 其余标本送常规病理检查. 采用免疫组化(SABC)方法检测胃癌组织、远离癌肿正常胃组织中Axin蛋白的表达. 两位专业病理医师双盲评估Axin表达平均值, 染色评估时隐藏临床生物学特性, 评估相差2分以上则重新进行评估. 依据Nakajima et al[9]的判断标准修订了组织表达阳性和阴性的标准. 根据细胞染色强度和染色细胞所占面积积分之和进行判定: (1)色强度积分: 未染色为0分; 轻度染色为1分; 中度染色为2分; 强染色为3分. (2) 染色面积积分: 无细胞染色为0分, 细胞染色<25%为1分; 细胞染色25%-50%为2分; 细胞染色>50%为3分. 若两项积分之和≥2为表达阳性; <2为表达阴性. 临床病理分期采用2003年UICC和AJCC制定的胃癌TNM分期标准.
统计学处理 采用成组对照设计, 借助SPSS12.0统计软件, 两组计数资料采用卡方检验分析. 所有假设检验用双侧检验, 检验水准α=0.05, 以P<0.05认为差异有统计学意义.
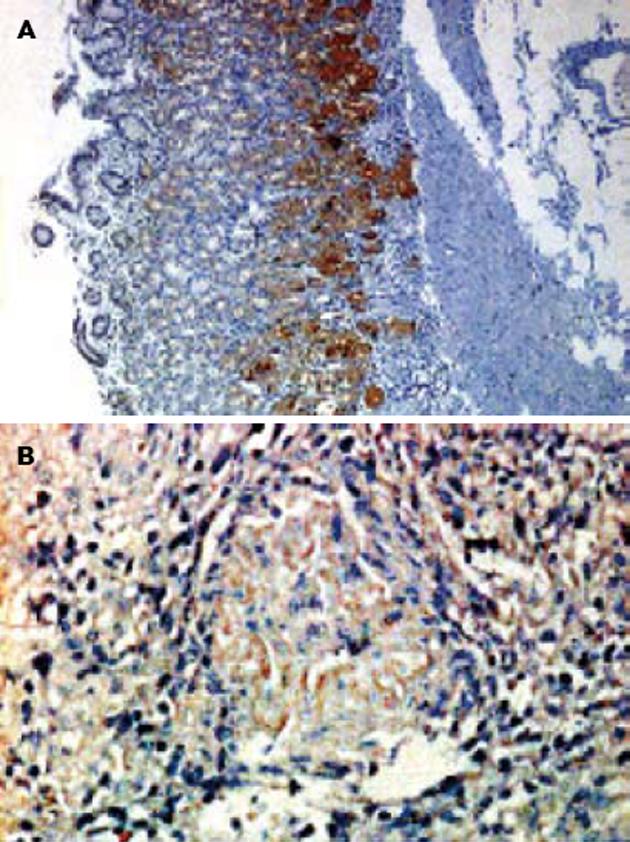
Axin蛋白染色成棕黄色, 主要分布在细胞质中, 分布比较均匀. 远癌正常组织与胃癌组织作比较发现, Axin蛋白在胃黏膜基底部位分裂增生能力较强的细胞中的表达较强, 在黏膜表面的细胞中表达较弱(图1A), 胃癌组织中Axin蛋白的表达则明显减弱(图1B). Axin蛋白在胃癌组织(62.0%)和正常胃组织中(91.3%)的表达率差异有统计学意义(P<0.01). Axin蛋白表达的减弱与胃癌的发生有相关性.
进一步亚组分析Axin蛋白表达与胃癌临床TNM分期、有无淋巴转移有一定的相关性(P<0.05), 与患者的性别、年龄、肿瘤大小、生物学特征和是否侵及浆膜无关(P>0.05, 表1).
| 病理特征 | n | Axin蛋白阳性n(%) | P值 |
| 性别 | |||
| 男 | 33 | 18(54.5) | 0.343 |
| 女 | 13 | 11(84.6) | |
| 年龄(岁) | 0.885 | ||
| <50 | 36 | 22(61.1) | |
| ≥50 | 10 | 7(70.0) | |
| 有无淋巴转移 | 0.034 | ||
| 无 | 14 | 12(85.7) | |
| 有 | 32 | 17(53.1) | |
| 肿瘤TNM分期 | 0.035 | ||
| Ⅰ Ⅱ | 14 | 11(78.6) | |
| Ⅲ Ⅳ | 32 | 18(56.3) | |
| 肿瘤直径(cm) | 0.558 | ||
| <5 | 30 | 18(60.0) | |
| ≥5 | 16 | 11(68.8) | |
| 分化 | 0.989 | ||
| 中、高 | 19 | 12(63.2) | |
| 低 | 27 | 17(63.0) | |
| 侵及浆膜 | 0.376 | ||
| 否 | 13 | 10(76.9) | |
| 是 | 33 | 19(57.6) |
肿瘤是机体在各种致瘤因素作用下, 激活癌基因和/或灭活抑癌基因, 在基因水平上失去对细胞生长的正常调控而导致的异常增生. Axin基因是在研究小鼠融合基因时发现的一个抑癌基因[1], 人类位于染色体16p13.3, 编码的Axin蛋白是由832或956个氨基酸残基组成的支架蛋白, 与axil[10]和conductin[11]是同源蛋白, 具有多种蛋白结合位点, 参与体内多条信号传导途径的调控过程, 调节细胞的增殖和生长. Axin蛋白可以在RanGTP和输出因子CRM1的Ð同帮助下进行细胞内核浆穿梭, 将细胞核内的β-catenin携带到细胞质中, 降低细胞核内β-catenin的浓度[12]; Axin蛋白与APC、GSK-3 β结合成复合体后加速β-catenin的降解, 从而对Wnt信号传导途径起负向调控作用[13]; Axin蛋白与MEKK1结合并激活其下游因子MEKK4与MEKK7, 促进JNK信号传导途径的活化[14]; 激活JNK信号传导系统和降低β-catenin细胞内含量可以诱导多种细胞的凋亡[14]. Axin蛋白还可以通过HIPK2激活抑癌基因p53的蛋白表达[15]. 我们研究发现, Axin蛋白在正常胃黏膜基底部分裂增生能力强的细胞中的表达明显高于黏膜表面的成熟细胞. 成熟并正常生长的机体细胞如正常结肠上皮细胞中, Wnt信号传导途径处于非活化状态, 增殖分化活动相对静止[16]; 体内具有分裂增生能力的干细胞如胃黏膜基底细胞、骨髓造血细胞等, Wnt信号传导途径活化, 使干细胞保持未分化状态并进一步增殖生长[17], 对细胞增殖和分化过程起着重要的作用. 作为Wnt信号传导途径中的重要负向调控因子, Axin蛋白在具有较强增生能力的干细胞中含量的增加对维持其状态的稳定具重要意义.
Axin蛋白表达异常与多种肿瘤的发生、发展有密切关系. Song et al[18]发现在Axin基因突变时, 卵巢滤泡细胞的增殖能力会发生明显的增强. Axin蛋白表达异常导致肿瘤发生的机制目前尚不完全清楚, 可能通过Wnt信号途径、JNK信号传导途径以及对p53表达的作用参与肿瘤的发生. (1)Axin蛋白表达减少, 其抑制Wnt信号途径中与肿瘤发生有密切关系的靶基因(c-myc、cyclin D1、MMP-7等)激活的作用可能减弱[19]; (2)Axin蛋白表达减少时对p53的活化作用减弱或消失[15]; (3)Axin蛋白表达减少可能通过JNK信号传导途径和增加β-catenin胞内含量, 使诱导细胞凋亡的能力下降而导致肿瘤. 我们发现胃癌分期和淋巴转移与Axin蛋白表达相关, 与Nakajima et al[9]在食管癌研究的发现相似, 机制不清, 有学者认为可能与β-catenin[20]作用有关. 表达异常的β-catenin减少可促进血管内皮生长因子(vascular endothelial growth factor, VEGF)的表达[21], 刺激新生肿瘤血管生成所致, 诱导多种实体肿瘤细胞的转移[22].
通过对Axin蛋白表达的调控可能成为肿瘤治疗的新的靶点, Satoh et al[6]研究发现腺病毒载体导入野生的Axin基因后, 肝癌细胞中β-catenin的量明显降低, 细胞凋亡率增加, 这为肿瘤基因治疗的深入研究提供了方向. 同时, Nakajima et al[9]发现81例没有Axin基因突变的食管癌癌组织中, 有5例Axin蛋白表达明显减少, 除基因突变造成的Axin表达异常外, 是否存在其他因素调控Axin基因表达, 还需要进一步深入研究.
Wnt信号传导途径在调控细胞增殖、分化、运动性以及形态学等方面起着重要的作用. Axin蛋白是Wnt信号途径的重要的负向调控因子, Axin基因被认为是抑癌基因. 目前研究显示多种肿瘤的发生和某些肿瘤的浸润转移与Axin基因异常有密切关系, 但对Axin蛋白表达异常与肿瘤发生、发展的关系研究较少.
研究发现了正常胃组织中, Axin蛋白在其基底部细胞表达强, 表面成熟细胞中表达较弱的特点, 提出Axin蛋白在具有较强增生繁殖能力的专能干细胞中含量增加, 并系统研究了Axin蛋白的表达与胃癌临床特征的关系.
本研究对于Axin蛋白在胃癌的发生和侵袭转移中的作用提供了一定的研究结果. 为进一步进行肿瘤发生和转移机制的研究提供思路, 为进一步动物和临床肿瘤诊断和治疗提供理论的依据.
本研究利用免疫组化方法研究了Axin蛋白在正常胃黏膜组织中的表达和分布特点, 并进一步阐明了其与胃癌发生和侵袭转移的关系, 研究设计合理, 结果明确, 结论分析论述有据, 对于Axin蛋白在胃癌的发生和侵袭转移中的作用提供了一定的研究结果.
电编: 李琪 编辑:潘伯荣
| 1. | Zeng L, Fagotto F, Zhang T, Hsu W, Vasicek TJ, Perry WL 3rd, Lee JJ, Tilghman SM, Gumbiner BM, Costantini F. The mouse Fused locus encodes Axin, an inhibitor of the Wnt signaling pathway that regulates embryonic axis formation. Cell. 1997;90:181-192. [PubMed] [DOI] |
| 2. | Akiyama T. Wnt/beta-catenin signaling. Cytokine Growth Factor Rev. 2000;11:273-282. [PubMed] [DOI] |
| 3. | He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509-1512. [PubMed] |
| 4. | Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422-426. [PubMed] [DOI] |
| 5. | Webster MT, Rozycka M, Sara E, Davis E, Smalley M, Young N, Dale TC, Wooster R. Sequence variants of the axin gene in breast, colon, and other cancers: an analysis of mutations that interfere with GSK3 binding. Genes Chromosomes Cancer. 2000;28:443-453. [PubMed] [DOI] |
| 6. | Satoh S, Daigo Y, Furukawa Y, Kato T, Miwa N, Nishiwaki T, Kawasoe T, Ishiguro H, Fujita M, Tokino T. AXIN1 mutations in hepatocellular carcinomas, and growth suppression in cancer cells by virus-mediated transfer of AXIN1. Nat Genet. 2000;24:245-250. [PubMed] [DOI] |
| 7. | Wu R, Zhai Y, Fearon ER, Cho KR. Diverse mechanisms of beta-catenin deregulation in ovarian endometrioid adenocarcinomas. Cancer Res. 2001;61:8247-8255. [PubMed] |
| 8. | Dahmen RP, Koch A, Denkhaus D, Tonn JC, Sorensen N, Berthold F, Behrens J, Birchmeier W, Wiestler OD, Pietsch T. Deletions of AXIN1, a component of the WNT/wingless pathway, in sporadic medulloblastomas. Cancer Res. 2001;61:7039-7043. [PubMed] |
| 9. | Nakajima M, Fukuchi M, Miyazaki T, Masuda N, Kato H, Kuwano H. Reduced expression of Axin correlates with tumour progression of oesophageal squamous cell carcinoma. Br J Cancer. 2003;88:1734-1739. [PubMed] [DOI] |
| 10. | Yamamoto H, Kishida S, Uochi T, Ikeda S, Koyama S, Asashima M, Kikuchi A. Axil, a member of the Axin family, interacts with both glycogen synthase kinase 3beta and beta-catenin and inhibits axis formation of Xenopus embryos. Mol Cell Biol. 1998;18:2867-2875. [PubMed] [DOI] |
| 11. | Behrens J, Jerchow BA, Wurtele M, Grimm J, Asbrand C, Wirtz R, Kuhl M, Wedlich D, Birchmeier W. Functional interaction of an axin homolog, conductin, with beta-catenin, APC, and GSK3beta. Science. 1998;280:596-599. [PubMed] [DOI] |
| 12. | Wiechens N, Heinle K, Englmeier L, Schohl A, Fagotto F. Nucleo-cytoplasmic shuttling of Axin, a negative regulator of the Wnt-beta-catenin Pathway. J Biol Chem. 2004;279:5263-5267. [PubMed] [DOI] |
| 13. | Ikeda S, Kishida S, Yamamoto H, Murai H, Koyama S, Kikuchi A. Axin, a negative regulator of the Wnt signaling pathway, forms a complex with GSK-3beta and beta-catenin and promotes GSK-3beta-dependent phosphorylation of beta-catenin. EMBO J. 1998;17:1371-1384. [PubMed] [DOI] |
| 14. | Neo SY, Zhang Y, Yaw LP, Li P, Lin SC. Axin-induced apoptosis depends on the extent of its JNK activation and its ability to down-regulate beta-catenin levels. Biochem Biophys Res Commun. 2000;272:144-150. [PubMed] [DOI] |
| 15. | Rui Y, Xu Z, Lin S, Li Q, Rui H, Luo W, Zhou HM, Cheung PY, Wu Z, Ye Z. Axin stimulates p53 functions by activation of HIPK2 kinase through multimeric complex formation. EMBO J. 2004;23:4583-4594. [PubMed] [DOI] |
| 16. | Chen RH, McCormick F. Selective targeting to the hyperactive beta-catenin/T-cell factor pathway in colon cancer cells. Cancer Res. 2001;61:4445-4449. [PubMed] |
| 17. | Feng Z, Srivastava AS, Mishra R, Carrier E. A regulatory role of Wnt signaling pathway in the hematopoietic differentiation of murine embryonic stem cells. Biochem Biophys Res Commun. 2004;324:1333-1339. [PubMed] [DOI] |
| 18. | Song X, Xie T. Wingless signaling regulates the maintenance of ovarian somatic stem cells in Drosophila. Development. 2003;130:3259-3268. [PubMed] [DOI] |
| 19. | Takahashi M, Tsunoda T, Seiki M, Nakamura Y, Furukawa Y. Identification of membrane-type matrix metalloproteinase-1 as a target of the beta-catenin/Tcf4 complex in human colorectal cancers. Oncogene. 2002;21:5861-5867. [PubMed] [DOI] |
| 20. | Horvath LG, Henshall SM, Lee CS, Kench JG, Golovsky D, Brenner PC, O'Neill GF, Kooner R, Stricker PD, Grygiel JJ. Lower levels of nuclear beta-catenin predict for a poorer prognosis in localized prostate cancer. Int J Cancer. 2005;113:415-422. [PubMed] [DOI] |
| 21. | Zhang X, Gaspard JP, Chung DC. Regulation of vascular endothelial growth factor by the Wnt and K-ras pathways in colonic neoplasia. Cancer Res. 2001;61:6050-6054. [PubMed] |
| 22. | Muller T, Bain G, Wang X, Papkoff J. Regulation of epithelial cell migration and tumor formation by beta-catenin signaling. Exp Cell Res. 2002;280:119-133. [PubMed] [DOI] |