修回日期: 2005-12-01
接受日期: 2005-12-08
在线出版日期: 2006-01-28
磷脂酰肌醇3-激酶(PI3Ks)信号参与增殖、分化、凋亡和葡萄糖转运等多种细胞功能的调节. 近年来发现, IA型PI3K和其下游分子蛋白激酶B(PKB或Akt)所组成的信号通路与人类肿瘤的发生发展密切相关. 该通路调节肿瘤细胞的增殖和存活, 其活性异常不仅能导致细胞恶性转化, 而且与肿瘤细胞的迁移、黏附、肿瘤血管生成以及细胞外基质的降解等相关, 目前以PI3K-Akt信号通路关键分子为靶点的肿瘤治疗策略正在发展中.
引文著录: 孙晓杰, 黄常志. PI3K-Akt信号通路与肿瘤. 世界华人消化杂志 2006; 14(3): 306-311
Revised: December 1, 2005
Accepted: December 8, 2005
Published online: January 28, 2006
N/A
- Citation: N/A. N/A. Shijie Huaren Xiaohua Zazhi 2006; 14(3): 306-311
- URL: https://www.wjgnet.com/1009-3079/full/v14/i3/306.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v14.i3.306
磷脂酰肌醇3-激酶(PI3K)家族参与多种信号通路, 调节细胞的主要功能. 正常情况下, 由其活化而产生的类脂产物3,4-二磷酸磷脂酰肌醇[PI(3,4)P2]和3,4,5-三磷酸磷脂酰肌醇[PI(3,4,5)P3]作为第二信使结合并激活多种细胞内的靶蛋白, 形成一个信号级联复合物, 最终调节细胞的增殖、分化、存活和迁移等[1]. 在PI3K家族中, IA型PI3K和其下游分子丝氨酸/苏氨酸蛋白激酶Akt(或PKB)所组成的信号通路因其与肿瘤发生发展的相关性, 近年来备受瞩目. PI3K-Akt信号通路在广泛的人类肿瘤谱中失调, 该通路某些成分的突变所导致的功能获得或功能缺失能够引起细胞转化, 这条通路调节肿瘤细胞的增殖和存活, 与肿瘤的侵袭转移行为也密切相关, 目前以该通路为靶点的肿瘤治疗策略正在发展中. 我们就这方面的进展加以综述.
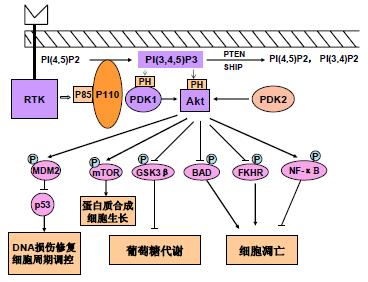
在PI3K家族中, 研究最广泛的是能被细胞表面受体所激活的I型PI3K. 哺乳动物细胞中Ι型PI3K又分为IA和IB两个亚型, 他们分别从酪氨酸激酶连接受体和G蛋白连接受体传递信号. IA 型PI3K是由催化亚单位p110和调节亚单位p85所组成的二聚体蛋白, 具有类脂激酶和蛋白激酶的双重活性[2]. PI3K通过两种方式激活, 一种是与具有磷酸化酪氨酸残基的生长因子受体或连接蛋白相互作用, 引起二聚体构象改变而被激活; 另一种是通过Ras和p110直接结合导致PI3K的活化[3]. PI3K激活的结果是在质膜上产生第二信使PIP3, PIP3与细胞内含有PH结构域的信号蛋白Akt和PDK1(phosphoinositide-dependent kinase-1)结合, 促使PDK1磷酸化Akt蛋白的Ser308导致Akt的活化. Akt还能通过PDK2(如整合素连接激酶ILK)对其Thr473的磷酸化而被激活[4]. 活化的Akt通过磷酸化作用激活或抑制其下游靶蛋白Bad 、Caspase9、NF-κB、GSK-3、FKHR、 p21Cip1和p27 Kip1等, 进而调节细胞的增殖、分化、凋亡以及迁移等(图1).
PI3K-Akt信号通路的活性被类脂磷酸酶PTEN(phosphatase and tensin homolog deleted on chromosome ten)和SHIP(SH2-containing inositol 5-phosphatase)负调节, 他们分别从PIP3的3'和5'去除磷酸而将其转变成PI(4,5)P2和PI(3,4)P2而降解[5]. 迄今为止, 尚未发现下调Akt活性的特异磷酸酶, 但用磷酸酶抑制剂处理细胞后, 发现Akt的磷酸化和活性均有所增加. 最近发现Akt能被一种C末端调节蛋白(CTMP)所失活, CTMP能结合Akt并通过抑制Akt的磷酸化而阻断下游信号的传递, CTMP的过表达能够逆转v-Akt转化细胞的表型. 热休克蛋白90(HSP90)亦能结合Akt, 阻止Akt被PP2A磷酸酶的去磷酸化而失活, 因此具有保护Akt的作用[4].
PI3K的活性与乳腺癌、肺癌、黑色素瘤和淋巴瘤等多种人类肿瘤的发生相关[6]. 据报道在人类肿瘤中, PI3K-Akt通路关键分子的编码基因在DNA水平上存在结构的变化, 如最初从人类肿瘤细胞系分离到的p85调节亚单位的突变体-截短的p65-PI3K(缺乏iSH2结构域和C末端的SH2结构域), 能引起PI3K的组成性活化和细胞转化; 大肠癌和卵巢癌中p85a基因在iSH2区域的缺失和突变能导致PI3K的激活[5,7]; 在非小细胞肺癌、前列腺腺癌和肠癌中亦存在PI3K、Akt、NF-kappaB/p65等持续活化以及表达量增高 [8-10]; 最近在Hodgkin's淋巴瘤分离到p85a基因的突变[11], 在乳腺癌、卵巢癌和胰腺癌等多种肿瘤中发现p110和Akt2编码基因突变或扩增[12-18], 而抑制PI3K活性后细胞的增殖明显被抑制, 表现为细胞周期阻滞、G1期相关蛋白CyclinD1和CDK4表达量减少以及 Rb的磷酸化等, 这种细胞周期的阻滞能因细胞中活性Akt及其下游分子p70S6K的表达而恢复. 上述情况与乳腺癌中非突变ERBB2RTK的扩增相类似, 由此推测结构正常的蛋白过表达可能导致转化.
在对人类肿瘤PTEN基因缺失或突变的调查中发现, PTEN缺失发生在广泛的人类肿瘤中[2], PTEN功能的缺失可以通过转录沉默或蛋白不稳定而发生. 这表明PI3K-Akt信号通路中的关键分子如p110a、p85a、Akt和PTEN等编码基因的结构改变与细胞转化相关, 这些基因目前已被证实是原癌基因或肿瘤抑制基因, 推测这些基因结构的改变使PI3K活化过程中通过正常RTK(receptor tyrosine kinase)信号对p85/p110复合物的负调节作用被解除, 从而导致PI3K的组成性活化和细胞转化. 目前已经在小鼠中建立了该通路异常的多种动物模型, 并且在有些模型中确实已经观察到该通路异常所导致的细胞转化和肿瘤发生.
细胞凋亡是控制过度增殖的一种正常细胞功能, 癌细胞具有多种抑制凋亡、延长其存活的机制. Akt主要作用于抗凋亡通路, 因为显性负效应Akt能阻止由类胰岛素生长因子1(IGF1)介导的存活, 持续激活Akt能够阻止由PTEN介导的凋亡. Akt通过对其下游的靶蛋白进行磷酸化而发挥其抗凋亡的作用, 如Akt能通过磷酸化Bcl-2家组成员BAD和蛋白水解酶Caspase-9而阻止凋亡; 还能磷酸化转录因子Forkhead家族成员FKHR, 通过抑制FKHR的核转位以及FKHR的基因靶点BIM和FAS配体的激活而抑制凋亡; 此外, Akt还能通过对NF-κB和P53的间接作用影响细胞存活[19], Akt通过磷酸化激活kB激酶(IKK)导致NF-κB的抑制剂IkB的降解, 从而使NF-κB从细胞质中释放出来进行核转位, 激活其靶基因而促进细胞的存活. 最近发现, Akt能通过磷酸化P53结合蛋白MDM2影响P53的活性, 磷酸化的MDM2转位到细胞核与P53结合, 通过增加P53蛋白的降解而影响细胞存活.
细胞周期被细胞周期蛋白依赖性激酶(CDK)复合物和CDK抑制剂(CDIs)的协调作用所调节, Akt通过调节细胞周期影响细胞增殖. Cyclin D1的水平对细胞周期G1/S的转换至关重要, GSK3b通过磷酸化作用促进泛素介导的Cyclin D1的降解, Akt通过直接磷酸化抑制GSK3b的激酶活性从而阻止Cyclin D1的降解. Akt还对CKIs如p27Kip1和p21Cip1的表达具有负调节作用, 他通过影响p21Cip1的磷酸化和与PCNA结合而调节p21Cip1的活性, 导致细胞增殖的增加[8]. Akt还能直接磷酸化p27Kip1的Thr157, 导致p27Kip1在细胞质中的滞留, 防止由p27Kip1所导致的细胞周期的阻滞[20]. 此外, Akt还能通过丝氨酸/苏氨酸激酶mTOR(the mammalian target of rapamycin)影响细胞存活和增殖, mTOR能激活核糖体激酶p70S6K(ribosomal S6-kinase, RSK), p70S6K通过抑制mRNA的转录后阻遏物4E-BP-1而增加mRNA的转录, 进而调节蛋白质的合成. 研究发现mTOR是Akt的直接靶点, 其活性能被PI3K 的抑制剂wortmannin和LY294002所抑制, 但mTOR被Akt磷酸化是否为其活化机制还不清楚[2]. 在肿瘤中, PI3K-Akt通路可能不是导致mTOR激活的唯一途径, 而且细胞的生长还可能通过PDK1与RSK的直接联系而不依赖于PI3K-Akt信号的调节.
肿瘤细胞的迁移和黏附在肿瘤侵袭转移中起着重要的作用. PI3K能传递整合素所介导的侵袭信号, 尤其对整合素a2b1、a6b4和aVb3的侵袭行为是必要的, 如PI3K介导前列腺癌中整合素aVb3驱动的侵袭特性, 在乳腺癌和卵巢癌中, Akt2的过表达能通过IV型胶原蛋白上调整合素b1从而增加细胞的侵袭和转移[21]; 但在c-erbB2所诱导的基质黏附和形成的破坏中, Akt显示出对整合素a2b1的功能起负调节作用[22]. 而在鳞癌细胞系中Akt的持续性表达能诱导上皮间质转变(epithelial mesenchymal transition, EMT), 赋予了组织侵袭和转移所需的运动性[23]. 表明PI3K-Akt通路参与了黏附因子所介导的细胞黏附、运动和侵袭作用.
肿瘤血管生成是肿瘤发生转移的必要条件. PI3K通过与E-Cadherin 、β-catenin和VEGFR-2形成复合物经由PI3K-Akt通路的活化参与血管内皮生长因子(VEGF)介导的内皮信号传递; 而VEGFR-2与aVb3复合物也以PI3K依赖的方式介导内皮细胞的黏附和迁移[1,24,25]. PI3K-Akt信号还能促进肿瘤坏死因子(TNF)诱导的内皮细胞迁移, 调节肿瘤血管的生成[26]. 基质金属蛋白酶(MMPs)和环氧化酶-2(COX-2)均能影响肿瘤血管生成, 在肿瘤的侵袭和转移中, 血小板衍生生长因子 (PDGF) 经由PI3K介导的通路诱导MMP3的表达 [27], 而上调抗凋亡蛋白Bcl-2和激活PI3K-Akt通路是COX-2刺激内皮细胞迁移和血管生成的主要机制[28,29]. 可见PI3K-Akt信号通路参与了多种因子所介导的肿瘤血管生成过程.
尿激酶型纤维蛋白酶原活性因子(uPA)是一个丝氨酸蛋白水解酶, 他能引起细胞外基质和基膜的重塑而促进肿瘤转移. uPA水平的增加与肿瘤细胞的侵袭特性密切相关, 如在肝细胞癌中uPA的上调与血管入侵、直接肝侵袭等密切相关, 在大肠癌中uPA的表达增加了从腺瘤发展到癌变的进程[30-33]. 近年来发现, PI3K在促进uPA介导的多种肿瘤细胞侵袭中起着重要的作用, 如在乳腺癌中uPA以依赖于PI3K和ERK的方式被ILGF-1上调, uPA的过表达是乳腺癌预后的强指示剂. 抑制PI3K信号能够抑制uPA的分泌, 导致乳腺癌的运动和迁移被抑制[34-39]. 转化生长因子b(TGF b)能以剂量和时间依赖的方式上调uPA mRNA和蛋白的表达促进转移, 而其上调uPA的作用也主要是通过PI3K/Akt/NF-κB通路所介导的[40]. 目前已经证实, uPA是NF-κB的靶基因, 因此推测PI3K-Akt途径的活化导致NF-κB的上调, 后者作用于其靶基因uPA 导致肿瘤的转移.
PI3K和其下游分子所转导的抗凋亡信号已经成为药物研究领域的焦点, 目前用于抑制该信号通路的策略主要有以下几个方面:
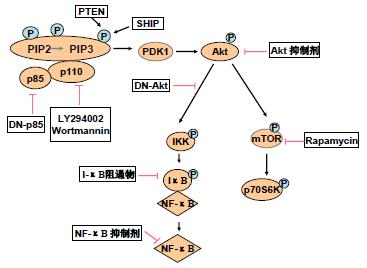
目前已经发现了该信号通路中多种激酶的小分子抑制剂(small molecule inhibitors, SMIs), Wortmannin和LY294002是两种广泛应用的PI3K抑制剂, 他们特异性抑制PI3Kp110亚单位的催化活性, 但这两种抑制剂因其对细胞的毒性作用目前还都限于体外研究, 尚未发展到临床应用. 最近又发现了Akt的抑制剂1L-6-hydroxymethyl-chiro-inositol 2-β-2-O-methyl-3-O-octadecylcarbonate, 其选择性抑制Akt的IC50值大约是5 mm, 明显小于其抑制PI3K的IC50值90 mm [41]; 酪氨酸磷酸化抑制剂AG597是Bcr-Abl染色体转位基因产物的特异抑制剂, 与慢性粒细胞性白血病的发展相关, 研究发现AG597能导致Akt去磷酸化而失活[42]; 最近发现KP372-1能够抑制甲状腺癌细胞中Akt的活化以及细胞增殖诱导细胞凋亡[43]. p70S6K是PI3K-Akt信号通路的另一可行性靶点, 免疫抑制剂Rapamycin(RPM)广泛应用于临床器官移植中, 研究发现, RPM能够使p70S6K去磷酸化而抑制该激酶的活性, 从而抑制肿瘤细胞的生长, 目前已经在多种PTEN突变和/或PI3K-Akt通路活性上调的人类肿瘤细胞系中观察到RPM的选择性抗肿瘤活性[44-46].
用基于质粒的反义载体进行转染或用人工合成的反义寡核苷酸进行治疗, 也已经用于干扰PI3K-Akt信号通路. 据报道, 反义cDNA的表达或用反义寡核苷酸治疗能下调p85基因的表达并消除PI3K信号, 反义RNA使过表达Akt2的PCNC1细胞在裸鼠中形成肿瘤的能力明显下降. Akt1的反义寡核苷酸在癌细胞中具有使细胞在软琼脂中的生长能力下降、诱导凋亡以及增加细胞对化疗药物敏感性等多种作用, 在PDK1的反义寡核苷酸研究中也得到了相似的结果[6].
显性负效应(domi-nant negative, DN)蛋白结合和抑制内源性蛋白的功能, Sonoyama et al[47]报道, p85调节亚单位的显性负效应蛋白Δp85的表达使Bcr-Abl转化细胞的细胞生长速度明显下降, p110显性负效应蛋白的作用亦有报道. Mabuchi et al [48]报道, 显性负效应Akt蛋白的表达使卵巢癌细胞对化疗药紫杉醇的敏感性增加; 最近发现, 显性负效应Akt能明显抑制肿瘤细胞的运动性[49], 这表明显性负效应Akt的表达能在一定程度上抑制肿瘤细胞的侵袭和转移. 此外, PI3K的信号还能通过提高磷酸酶PTEN或SHIP的活性而消除[50]. 抑制PI3K-Akt信号通路的策略见图2.
总之, PI3K-Akt信号通路对于细胞增殖、分化和凋亡的调节是必要的. 其组成性活化与肿瘤发生及肿瘤侵袭转移的相关性, 提示发展小分子药物有效抑制该通路的高活性, 将有可能提高恶性肿瘤临床治疗的效果. 目前, 对该信号通路在肿瘤侵袭转移中的作用机制还不清楚, 相信随着对这条通路分子机制研究的深入, 在不久的将来, 以PI3K-Akt通路为靶点的小分子药物将有望应用于肿瘤的临床治疗中.
PI3K-Akt信号通路调节细胞的增殖、分化、存活和迁移等功能. 近年来发现, 这条通路的活性异常不仅能导致细胞恶性转化, 还与肿瘤细胞的侵袭转移行为相关. 目前以这条信号通路为靶点的肿瘤治疗策略正在广泛的研究中.
本文简要介绍了PI3K-Akt通路的组成、活化和调节, 重点阐明该通路与肿瘤细胞转化、增殖以及转移的相关性, 并对当前以该通路为靶点的抑制策略加以总结.
本文阐明了PI3K-Akt信号通路与肿瘤发生发展的相关性, 为开发以该信号通路为靶点的小分子药物通过抑制该信号通路的活性进行肿瘤治疗提供了理论基础.
显性负效应(DN): 抑癌基因突变的拷贝在另一野生型拷贝存在并表达的情况下, 仍可使细胞出现恶性表型和癌变, 并使野生型拷贝功能失活, 这种作用称为显性负效应.
本文表达了PI3K-Akt信号通路的组成, 信号的途径及调控网络, 具有分子生物学的理论意义, 文中还强调了该信号通路与肿瘤发生、发展及转移潜能的关系, 提示了以信号传导异常为治疗靶点的策略及研究结论, 有较为广泛的可读性及启示.
编辑: 菅鑫妍 审读: 张海宁 电编: 张勇
| 1. | Katso R, Okkenhaug K, Ahmadi K, White S, Timms J, Waterfield MD. Cellular function of phosphoinositide 3-kinases: implications for development, homeostasis, and cancer. Annu Rev Cell Dev Biol. 2001;17:615-675. [PubMed] |
| 2. | Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489-501. [PubMed] |
| 3. | Ward SG, Finan P. Isoform-specific phosphoinositide 3-kinase inhibitors as therapeutic agents. Curr Opin Pharmacol. 2003;3:426-434. [PubMed] |
| 4. | Solit DB, Basso AD, Olshen AB, Scher HI, Rosen N. Inhibition of heat shock protein 90 function down-regulates Akt kinase and sensitizes tumors to Taxol. Cancer Res. 2003;63:2139-2144. [PubMed] |
| 5. | Jimenez C, Jones DR, Rodriguez-Viciana P, Gonzalez-Garcia A, Leonardo E, Wennstrom S, von Kobbe C, Toran JL, R-Borlado L, Calvo V. Identification and characterization of a new oncogene derived from the regulatory subunit of phosphoinositide 3-kinase. EMBO J. 1998;17:743-753. [PubMed] |
| 6. | Chang F, Lee JT, Navolanic PM, Steelman LS, Shelton JG, Blalock WL, Franklin RA, McCubrey JA. Involvement of PI3K/Akt pathway in cell cycle progression, apoptosis, and neoplastic transformation: a target for cancer chemotherapy. Leukemia. 2003;17:590-603. [PubMed] |
| 7. | Philp AJ, Campbell IG, Leet C, Vincan E, Rockman SP, Whitehead RH, Thomas RJ, Phillips WA. The phosphatidylinositol 3'-kinase p85alpha gene is an oncogene in human ovarian and colon tumors. Cancer Res. 2001;61:7426-7429. [PubMed] |
| 8. | Lee SH, Kim HS, Park WS, Kim SY, Lee KY, Kim SH, Lee JY, Yoo NJ. Non-small cell lung cancers frequently express phosphorylated Akt; an immunohistochemical study. APMIS. 2002;110:587-592. [PubMed] |
| 9. | Itoh N, Semba S, Ito M, Takeda H, Kawata S, Yamakawa M. Phosphorylation of Akt/PKB is required for suppression of cancer cell apoptosis and tumor progression in human colorectal carcinoma. Cancer. 2002;94:3127-3134. [PubMed] |
| 10. | Shukla S, Maclennan GT, Marengo SR, Resnick MI, Gupta S. Constitutive activation of PI3K-Akt and NF-kappaB during prostate cancer progression in autochthonous transgenic mouse model. Prostate. 2005;64:224-239. [PubMed] |
| 11. | Jucker M, Sudel K, Horn S, Sickel M, Wegner W, Fiedler W, Feldman RA. Expression of a mutated form of the p85alpha regulatory subunit of phosphatidylinositol 3-kinase in a Hodgkin×s lymphoma-derived cell line(CO). Leukemia. 2002;16:894-901. [PubMed] |
| 12. | Shayesteh L, Lu Y, Kuo WL, Baldocchi R, Godfrey T, Collins C, Pinkel D, Powell B, Mills GB, Gray JW. PIK3CA is implicated as an oncogene in ovarian cancer. Nat Genet. 1999;21:99-102. [PubMed] |
| 13. | Gao N, Flynn DC, Zhang Z, Zhong XS, Walker V, Liu KJ, Shi X, Jiang BH. G1 cell cycle progression and the expression of G1 cyclins are regulated by PI3K/AKT/mTOR/p70S6K1 signaling in human ovarian cancer cells. Am J Physiol Cell Physiol. 2004;287:C281-C291. [PubMed] |
| 14. | Saal LH, Holm K, Maurer M, Memeo L, Su T, Wang X, Yu JS, Malmstrom PO, Mansukhani M, Enoksson J. PIK3CA mutations correlate with hormone receptors, node metastasis, and ERBB2, and are mutually exclusive with PTEN loss in human breast carcinoma. Cancer Res. 2005;65:2554-2559. [PubMed] |
| 15. | Levine DA, Bogomolniy F, Yee CJ, Lash A, Barakat RR, Borgen PI, Boyd J. Frequent mutation of the PIK3CA gene in ovarian and breast cancers. Clin Cancer Res. 2005;11:2875-2878. [PubMed] |
| 16. | Hartmann C, Bartels G, Gehlhaar C, Holtkamp N, von Deimling A. PIK3CA mutations in glioblastoma multiforme. Acta Neuropathol (Berl). 2005;109:639-642. [PubMed] |
| 17. | Wu G, Mambo E, Guo Z, Hu S, Huang X, Gollin SM, Trink B, Ladenson PW, Sidransky D, Xing M. Uncommon mutation, but common amplifications, of the PIK3CA gene in thyroid tumors. J Clin Endocrinol Metab. 2005;90:4688-4693. [PubMed] |
| 18. | Ikenoue T, Kanai F, Hikiba Y, Obata T, Tanaka Y, Imamura J, Ohta M, Jazag A, Guleng B, Tateishi K. Functional analysis of PIK3CA gene mutations in human colorectal cancer. Cancer Res. 2005;65:4562-4567. [PubMed] |
| 19. | Jeong SJ, Pise-Masison CA, Radonovich MF, Park HU, Brady JN. Activated AKT regulates NF-kappaB activation, p53 inhibition and cell survival in HTLV-1-transformed cells. Oncogene. 2005;24:6719-6728. [PubMed] |
| 20. | Viglietto G, Motti ML, Bruni P, Melillo RM, D'Alessio A, Califano D, Vinci F, Chiappetta G, Tsichlis P, Bellacosa A. Cytoplasmic relocalization and inhibition of the cyclin-dependent kinase inhibitor p27(Kip1) by PKB/Akt-mediated phosphorylation in breast cancer. Nat Med. 2002;8:1136-1144. [PubMed] |
| 21. | Arboleda MJ, Lyons JF, Kabbinavar FF, Bray MR, Snow BE, Ayala R, Danino M, Karlan BY, Slamon DJ. Overexpression of AKT2/protein kinase Bbeta leads to up-regulation of beta1 integrins, increased invasion, and metastasis of human breast and ovarian cancer cells. Cancer Res. 2003;63:196-206. [PubMed] |
| 22. | Lindberg LE, Hedjazifar S, Baeckstrom D. c-erbB2-induced disruption of matrix adhesion and morphogenesis reveals a novel role for protein kinase B as a negative regulator of alpha(2)beta(1) integrin function. Mol Biol Cell. 2002;13:2894-2908. [PubMed] |
| 23. | Grille SJ, Bellacosa A, Upson J, Klein-Szanto AJ, van Roy F, Lee-Kwon W, Donowitz M, Tsichlis PN, Larue L. The protein kinase Akt induces epithelial mesenchymal transition and promotes enhanced motility and invasiveness of squamous cell carcinoma lines. Cancer Res. 2003;63:2172-2178. [PubMed] |
| 24. | Namiecinska M, Marciniak K, Nowak JZ. VEGF as an angiogenic, neurotrophic, and neuroprotective factor. Postepy Hig Med Dosw. 2005;59:573-583. [PubMed] |
| 25. | Tan PH, Xue SA, Manunta M, Beutelspacher SC, Fazekasova H, Shamsul Alam AK, McClure MO, George AJ. Effect of vectors on human endothelial cell signal transduction. implications for cardiovascular gene therapy. Arterioscler Thromb Vasc Biol. 2005;[PMID:14532277] 26 Zhang R, Xu Y, Ekman N, Wu Z, Wu J, Alitalo K, Min W. Etk/Bmx transactivates vascular endothelial growth factor 2 and recruits phosphatidylinositol 3-kinase to mediate the tumor necrosis factor-induced angiogenic pathway. J Biol Chem 2003; 278: 51267-51276. [PubMed] |
| 26. | Kanaki T, Bujo H, Mori S, Yanjuan Z, Takahashi K, Yokote K, Morisaki N, Saito Y. Functional analysis of aortic endothelial cells expressing mutant PDGF receptors with respect to expression of matrix metalloproteinase-3. Biochem Biophys Res Commun. 2002;294:231-237. [PubMed] |
| 27. | Gately S, Kerbel R. Therapeutic potential of selective cyclooxygenase-2 inhibitors in the management of tumor angiogenesis. Prog Exp Tumor Res. 2003;37:179-192. [PubMed] |
| 28. | Thiel A, Heinonen M, Rintahaka J, Hallikainen T, Hemmes A, Dixon DA, Haglund C, Ristimaki A. Expression of cyclooxygenase-2 is regulated by GSK-3beta in gastric cancer cells. J Biol Chem. 2005;[PMID:11689575] 30 Sliva D, Rizzo MT, English D. Phosphatidylinositol 3-kinase and NF-kappaB regulate motility of invasive MDA-MB-231 human breast cancer cells by the secretion of urokinase-type plasminogen activator. J Biol Chem 2002; 277: 3150-3157. [PubMed] |
| 29. | Hiendlmeyer E, Regus S, Wassermann S, Hlubek F, Haynl A, Dimmler A, Koch C, Knoll C, van Beest M, Reuning U. Beta-catenin up-regulates the expression of the urokinase plasminogen activator in human colorectal tumors. Cancer Res. 2004;64:1209-1214. [PubMed] |
| 30. | Li P, Gao Y, Ji Z, Zhang X, Xu Q, Li G, Guo Z, Zheng B, Guo X. Role of urokinase plasminogen activator and its receptor in metastasis and invasion of neuroblastoma. J Pediatr Surg. 2004;39:1512-1519. [PubMed] |
| 31. | Chan CF, Yau TO, Jin DY, Wong CM, Fan ST, Ng IO. Evaluation of nuclear factor-kappaB, urokinase-type plasminogen activator, and HBx and their clinicopathological significance in hepatocellular carcinoma. Clin Cancer Res. 2004;10:4140-4149. [PubMed] |
| 32. | Agarwal A, Das K, Lerner N, Sathe S, Cicek M, Casey G, Sizemore N. The AKT/I kappa B kinase pathway promotes angiogenic/metastatic gene expression in colorectal cancer by activating nuclear factor-kappa B and beta-catenin. Oncogene. 2005;24:1021-1031. [PubMed] |
| 33. | Chandrasekar N, Mohanam S, Gujrati M, Olivero WC, Dinh DH, Rao JS. Downregulation of uPA inhibits migration and PI3k/Akt signaling in glioblastoma cells. Oncogene. 2003;22:392-400. [PubMed] |
| 34. | Kobayashi H, Suzuki M, Kanayama N, Terao T. Genetic down-regulation of phosphoinositide-3-kinase by bikunin correlates with suppression of invasion and metastasis in human ovarian cancer HRA cells. J Biol Chem. 2004;279:6371-6379. [PubMed] |
| 35. | Wang M, Vogel I, Kalthoff H. Correlation between metastatic potential and variants from colorectal tumor cellline HT-29. World J Gastroenterol. 2003;9:2627-2631. [PubMed] |
| 36. | Sliva D. Signaling pathways responsible for cancer cell invasion as targets for cancer therapy. Curr Cancer Drug Targets. 2004;4:327-336. [PubMed] |
| 37. | Kobayashi H, Suzuki M, Kanayama N, Terao T. Genetic down-regulation of phosphoinositide 3-kinase by bikunin correlates with suppression of invasion and metastasis in human ovarian cancer HRA cells. J Biol Chem. 2004;279:6371-6379. [PubMed] |
| 38. | Tanaka Y, Kobayashi H, Suzuki M, Kanayama N, Terao T. Transforming growth factor-beta1-dependent urokinase up-regulation and promotion of invasion are involved in Src-MAPK-dependent signaling in human ovarian cancer cells. J Biol Chem. 2004;279:8567-8576. [PubMed] |
| 39. | Hu Y, Qiao L, Wang S, Rong SB, Meuillet EJ, Berggren M, Gallegos A, Powis G, Kozikowski AP. 3-(Hydroxymethyl)-bearing phosphatidylinositol ether lipid analogues and carbonate surrogates block PI3-K, Akt, and cancer cell growth. J Med Chem. 2000;43:3045-3051. [PubMed] |
| 40. | Urbano A, Gorgun G, Foss F. Mechanisms of apoptosis by the tyrphostin AG957 in hematopoietic cells. Biochem Pharmacol. 2002;63:689-692. [PubMed] |
| 41. | Mandal M, Kim S, Younes MN, Jasser SA, El-Naggar AK, Mills GB, Myers JN. The Akt inhibitor KP372-1 suppresses Akt activity and cell proliferation and induces apoptosis in thyroid cancer cells. Br J Cancer. 2005;92:1899-1905. [PubMed] |
| 42. | Georgakis GV, Younes A. From Rapa Nui to rapamycin: targeting PI3K/Akt/mTOR for cancer therapy. Expert Rev Anticancer Ther. 2006;6:131-140. [PubMed] |
| 43. | Takeuchi H, Kondo Y, Fujiwara K, Kanzawa T, Aoki H, Mills GB, Kondo S. Synergistic augmentation of rapamycin-induced autophagy in malignant glioma cells by phosphatidylinositol 3-kinase/protein kinase B inhibitors. Cancer Res. 2005;65:3336-3346. [PubMed] |
| 44. | Avellino R, Romano S, Parasole R, Bisogni R, Lamberti A, Poggi V, Venuta S, Romano MF. Rapamycin stimulates apoptosis of childhood acute lymphoblastic leukemia cells. Blood. 2005;106:1400-1406. [PubMed] |
| 45. | Sonoyama J, Matsumura I, Ezoe S, Satoh Y, Zhang X, Kataoka Y, Takai E, Mizuki M, Machii T, Wakao H. Functional cooperation among Ras, STAT5, and phosphatidylinositol 3-kinase is required for full oncogenic activities of BCR/ABL in K562 cells. J Biol Chem. 2002;277:8076-8082. [PubMed] |
| 46. | Mabuchi S, Ohmichi M, Kimura A, Hisamoto K, Hayakawa J, Nishio Y, Adachi K, Takahashi K, Arimoto-Ishida E, Nakatsuji Y. Inhibition of phosphorylation of BAD and Raf-1 by Akt sensitizes human ovarian cancer cells to paclitaxel. J Biol Chem. 2002;277:33490-33500. [PubMed] |
| 47. | Okudela K, Hayashi H, Ito T, Yazawa T, Suzuki T, Nakane Y, Sato H, Ishi H, KeQin X, Masuda A. K-ras gene mutation enhances motility of immortalized airway cells and lung adenocarcinoma cells via Akt activation: possible contribution to non-invasive expansion of lung adenocarcinoma. Am J Pathol. 2004;164:91-100. [PubMed] |
| 48. | Fujita T, Doihara H, Kawasaki K, Takabatake D, Takahashi H, Washio K, Tsukuda K, Ogasawara Y, Shimizu N. PTEN activity could be a predictive marker of trastuzumab efficacy in the treatment of ErbB2-overexpressing breast cancer. Br J Cancer. 2006;94:247-252. [PubMed] |