修回日期: 2006-06-01
接受日期: 2006-06-05
在线出版日期: 2006-07-28
胰腺癌预后不良, 死亡率高居消化道癌症第5位. 现代医学对胰腺癌的病因尚未完全清楚, 但随着肿瘤发生、发展分子机制的逐步了解, 癌细胞与正常细胞的区别将会更加明确, 一些癌细胞特有的分子靶点将成为胰腺癌诊疗的有用工具. 基因检测和蛋白质检测, 以及手术治疗、放射治疗和化学治疗为本病常用的诊疗方法. 另一方面, 中医辨证施治、标本兼治、阴阳平衡等方法和理论, 如能恰当地融合到西医对胰腺癌的综合诊疗中, 定将提高治愈率, 并能改善患者的生存质量. 此外, 分子靶向治疗如免疫治疗、核糖核酸干扰等在近年来蓬勃兴起, 尽管其技术仍需改善, 疗效有待提高, 但已给恶性肿瘤的诊疗带来了希望. 本文对胰腺癌作出较详尽的介绍, 并阐述目前胰腺癌诊疗的最新进展.
引文著录: 曹志成. 中西医与分子靶向综合治疗胰腺癌的进展. 世界华人消化杂志 2006; 14(21): 2049-2054
Revised: June 1, 2006
Accepted: June 5, 2006
Published online: July 28, 2006
N/A
- Citation: N/A. N/A. Shijie Huaren Xiaohua Zazhi 2006; 14(21): 2049-2054
- URL: https://www.wjgnet.com/1009-3079/full/v14/i21/2049.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v14.i21.2049
胰腺癌预后不良, 其发病率近年有显着上升趋势. 据世界卫生组织2002年资料显示, 全球每年新发病例约23.2万, 为世界第13大常见恶性肿瘤, 死亡人数约22.7万, 为消化道癌症第5号杀手. 我国年发病人数约4.3万, 每年约有3.7万人死于此病, 死亡率占癌症中的第7位[1]. 据统计, 只有约15%病例可进行手术治疗, 5 a生存率不足3%, 超过90%患者在确诊后1 a内死亡, 平均存活期少于6 mo[2].
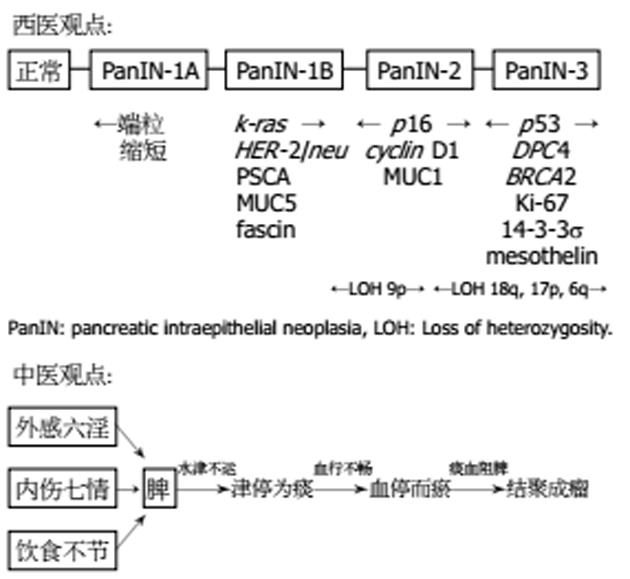
细现代医学对胰腺癌的病因尚未清楚, 西医认为本病的成因有: (1)吸烟. 吸烟者罹患胰腺癌是非吸烟者的2倍[3-4]; (2)经常进食高动物脂肪和蛋白、或含亚硝酸盐类食物者,属高危发病人群[5]; (3)1型糖尿病患者合并胰腺癌者较一般人高2倍[6]; (4)亦有认为胰腺癌与家族性慢性胰腺炎或胰腺结石有一定关系, 但目前尚未完全确定(图1). 胰腺癌属中医脾积、积聚、黄疸范畴, 其病位在脾, 中医认为本病的病因病机有: (1)外感湿毒, 损伤脾气, 脾运失司, 水湿不化, 郁而化热, 湿热内蕴, 酿毒结瘤; (2)脾居中州, 为气机升降之枢纽, 内伤忧思, 抑郁伤脾, 脾气郁结, 升降失常, 水津不运, 血行不畅, 津停为痰, 血停而瘀, 痰血阻脾, 结聚成瘤; (3)饮食不节, 内伤酒食, 伤脾损胃, 聚湿生痰, 痰湿瘀血结聚于脾, 日久不散, 酿生癌瘤(图1).
根据胰腺癌的发生部位, 可分为胰头癌、胰体癌、胰尾癌和全胰癌, 其临床表现隐匿, 且发病迅速, 常见症状有: (1)腹痛. 胰头癌常有右上腹痛, 胰体、胰尾癌多见中上腹痛或左上腹痛; (2)黄疸和皮肤瘙痒; (3)体质量下降. 常规诊断有: (1)胃肠X线钡餐检查、选择性血管造影(SAG)、纤维胃镜超声检查[7]或内镜下逆行胰胆管造影(ERCP), 有助于与胃部疾病区别; (2)血清免疫生化检测可将急慢性胰腺炎、肝炎、胆囊炎与本病相鉴别; (3)B超、增强螺旋CT扫描或磁共振成像(MRI), 可与胆石症、原发性肝癌、壶腹周围癌相鉴别; (4)正电子放射断层扫描(PET)采用定量或半定量方法计算胰腺癌组织中的18F-FDG含量, 有助于胰腺癌与慢性胰腺炎的鉴别诊断, 其敏感性可达94%, 特异性为88%, 但该方法因费用昂贵, 不适于常规临床应用; (5) 胰腺活组织检查可确诊高度怀疑的病例.
目前已发现一些与胰腺癌相关的肿瘤标记物, 但由于特异性偏低, 且对瘤体较小的病例阳性率低, 尚未被广泛应用, 临床常用的标记物有以下几个: (1)CA19-9: 其敏感性、特异性和准确性分别为83%, 73%和75%, 但小于2 cm的胰腺癌阳性率仅61%; (2)CA50: 与CA19-9同属Lewis系统抗原, 两者无需同时测定, 但CA50和CA242联合检测则可提高敏感性; (3)MIC-1: 与正常组比较, 其受试者作业特征(ROC)曲线评价结果高达0.99, 与慢性胰腺炎比较亦有0.81, 比CA19-9更具诊断价值[8]; (4)Span-1: 其敏感性和特异性分别为81%和68%, 但小于2 cm的胰腺癌阳性率仅56%; (5)Dupan-2: 敏感性约60%, 若与CA19-9联合检测阳性率可提高到95%; (6)CEA(癌胚抗原): 阳性率约79%, CEA阴性者平均生存期为39 mo, 可用以判断预后.
随着肿瘤发生、发展分子机制的阐明, 癌细胞与正常细胞的区别将会更加明确, 一些癌细胞特有的分子靶点将成为胰腺癌诊疗的有用工具[9]. (1)ras: 胰腺癌有高表达k-ras基因, 阳性率达90%, 而胰腺其他疾病不具有这种标志性改变, ras基因突变的检测, 可能成为早期诊断胰腺癌的可靠分子生物学手段[10-11]; (2)C-erbB-2和COX-2: 其基因产物在胰腺癌中呈高表达, 与肿瘤大小及预后有关[12]; (3)p16, p21和p53: 在胰腺癌中表达率高的抑癌基因, 具有特异性, 对良、恶性胰腺肿瘤具鉴别价值[13]; (4)Rap1GAP: 在1p35-p36上Rap1GAP的缺失与胰腺癌有关[14]; (5)PD2/hPaf1: 在19q13上PD2/hPaf1的高表达与胰腺癌的发展有关[15]; (6)S100A6: 采用微阵列芯片(microarray)检测胰腺液中S100A6信使核糖核酸, 可有效鉴别胰腺癌与非恶性肿瘤患者[16]; (7)RecQ1, RAD54L和ATM: 从单核甘酸多态性研究显示, 这些基因与胰腺癌生存期的缩短有关[17]; (8)甲基化检测: 利用甲基化特异性基因扩增(MSP)技术量化胰腺液中基因(如cyclin D2, FOXE1, NPTX2, ppENK, p16, Reprimo和TFPI2等)甲基化的变化, 可有助胰腺癌的诊断[18-19].
胰腺癌患者血清中IL-10, TGF-β, VEGF, PSCA和KU-CT-1呈高表达[20-23], 组织中EGFR亦呈高表达[24], 可作为免疫治疗的靶点; 应用质谱技术发现血清fibrinogen-γ[25]和自身免疫抗体DDX48[26]在胰腺癌中明显升高, 可作为与正常病例区分的生物标志; 用蛋白质组定量分析则发现, 载脂蛋白E, α-1-抗胰凝乳蛋白酶, inter-alpha-trypsin inhibitor, 组织蛋白酶D, 巨噬细胞集落刺激因子, 纤维连接因子受体, 抑制蛋白1, IGFBP-7和胰岛素样生长因子结合蛋白-2均与胰腺癌有关[27-29]; 亦有用表面增强激光解吸离子化-飞行时间质谱(SELDI-TOF MS)技术检测到一系列可能与胰腺癌相关的生物标志[30-31].
因胰腺癌早期缺乏特异性临床表现, 确诊时往往已届晚期, 治疗效果差, 死亡率高, 常用治疗手段分述如下.
近年肿瘤治疗越来越趋向综合治疗方向发展, 下列常规治疗手段除单独使用外, 更常合理地综合应用, 提高治愈机会[32]. (1)手术治疗: 为主要治疗方法, 胰头癌多采Whipple手术(PD), 壶腹周围癌或壶腹部癌可用保留幽门的胰十二指肠切除术(PPPD), 癌组织涉及全胰但无肝转移及腹膜种植者, 可采取全胰切除术, 胰体尾部无癌转移者可采用胰体尾切除术(DP), 癌组织与周围器官广泛浸润并向远处转移者, 可采用姑息性外科治疗缓解黄疸症状[33-34]; (2)放射治疗: 在术前、术中、术后或化疗期间施行放疗对胰腺癌有一定抑制作用, 可延长患者生存期[35-37]; (3)化学治疗: 虽然胰腺癌对化疗药物不敏感, 但化疗可延长生存期, 使部分病情得以缓解, 如以raltitrexed-oxaliplatin联合化疗来治疗单药gemcitabine耐药患者的胰腺癌转移[38], 实验显示trichostatin A联合其他化疗药物一同使用, 有增效作用[39], 临床试验证明口服药物erlotinib联合gemcitabine能延长晚期患者的生存期[40], 而gemcitabine联合epirubicin治疗晚期胰腺癌患者亦已进入Ⅱ期临床试验, 效果令人鼓舞[41], 其他常用联合化疗方案有FAM, FAP和MA等.
中医治疗胰腺癌讲求辨证论治, 按其个体临床症状可分为: (1)气滞血瘀、络脉瘀阻者, 方以金铃子散合失笑散加味, 用以行气活血、散结止痛; (2)痰瘀交凝、积聚于脾者, 方以膈下逐瘀汤合涤痰汤化裁, 用以涤痰化瘀、软坚散结; (3)邪毒内攻、胆火上炎者, 方以茵陈蒿汤合黄连解毒汤加减, 用以清热解毒、利胆泄浊; (4)气虚血弱、脉络瘀阻者, 方以十全大补汤加减, 用以益气养血、化瘀散结.
胰腺癌的治疗目前尚无新的突破, 仍以早期手术切除为首选, 但手术切除率不高, 确诊时大多数为中晚期患者, 因此适宜用个体化中西医结合治疗, 常用治疗方案有: (1)手术与中医药结合治疗. 根治手术后可以中药健脾和胃、益气养血, 仅作姑息性手术治疗者, 术后可用中药改善体质, 增强机体抵抗能力; (2)化疗与中医药结合治疗. 健脾益气中药可减轻化疗毒副反应, 如红、白细胞和血小板低下, 也可减少哆嗦、乏力等症状, 更能提高疗效; (3)手术、放疗与中医药结合治疗. 根治手术后采用放疗和长期以疏肝健脾中药治疗, 对提高远期生存具有一定意义; (4)手术、化疗与中医药结合治疗. 姑息性手术治疗后结合扶正固本中药和化疗药物, 对改善生存质量和延长生存期有一定帮助.
胰腺癌患者多伴有痛症, 且疼痛难忍, 痛时常不能平卧, 夜间加重, 影响进食和睡眠, 止痛方法有: (1)阿司匹林. 适用于轻至中度疼痛; (2)吗啡. 用于缓解中度至重度疼痛; (3)神经阻滞. 经皮或术中注射药物行内脏神经阻滞, 通过化学方法破坏腹腔神经节; (4)针刺. 体针可取三阴交、太神、公孙(双侧), 耳针可取交感、神门、三焦、脾穴(双侧); (5)中药. 徐长卿和雷公藤均具止痛效力, 可用水煎服.
目前用以治疗胰腺癌最常用的基因治疗方法, 是将靶向基因载体直接注射或导入体内的肿瘤组织, 进行局部性基因治疗, 近年更采用联合基因治疗, 以增强疗效, 由于能针对肿瘤内特有的基因变异情况进行修复或促使肿瘤细胞死亡, 基因治疗具有广阔的应用前景[42-44], 常用分子靶向治疗策略有: (1)抑癌基因治疗. 将具有正常功能的野生型抑癌基因(如p53, p21, Rb, Thoc1/p84等)转染至胰腺癌细胞中, 重建失活的抑癌基因功能, 恢复细胞的正常生长表型, 或诱导细胞凋亡, 从而达到控制胰腺癌细胞异常生长的目的[45]; (2)病毒基因治疗. 利用肿瘤细胞和正常细胞中抑癌基因(如p53, Rb等)的差别, 以蛋白质(如E1A, E1B等)缺陷的腺病毒导入胰腺癌组织, 病毒的大量繁殖可导致肿瘤细胞裂解死亡[46]; (3)自杀基因治疗. 把自杀基因(如TK, CD, mda-7/IL-24, PNP, XGPRT等)转染至胰腺癌细胞中, 能将前体药物(如ACV, GCV等)转化为对分裂细胞具杀伤作用的代谢产物, 从而特异性地杀伤胰腺癌细胞[47-48]; (4)修饰基因治疗. 把多药耐药基因(MDR)导入体内的造血干细胞, 使其具有比胰腺癌细胞更强的化疗药物耐受力, 可以提高临床化疗剂量和时间, 减轻对骨髓细胞的损害[49]; (5)核糖核酸干扰(RNAi). RNAi识别可以精确到一个核苷酸, 对由野生型点突变形成的癌基因(如ras, LSM-1, p53等)和蛋白质(如Mirk, MUC1等), 能够产生准确有效的封闭效果, 其他正常野生型基因则不受影响. 基因的突变在胰腺癌的发病及发展中均占有重要地位, 通过改变癌基因的表达, 可以杀灭肿瘤或抑制肿瘤生长, 而利用RNAi技术调控基因(如GADD45)和蛋白质(如X染色体相关联细胞凋亡蛋白抑制剂)的表达, 能控制胰腺癌细胞的增殖[50-54], 或减轻化疗抗药性[55], 近年更引进崭新的小型干扰核糖核酸(siRNA)技术, 以此调控notch-1信号途径减低NF-κB, VEGF和MMPs在胰腺癌细胞中的高表达, 可抑制其侵略和扩散[56], 亦有利用survivin基因特异siRNA抑制胰腺癌细胞的生长[57-59]; (6)免疫治疗. 将血管生成抑制剂(如angiostatin, bevacizumab[60-61], endostatin和PEDF[62]等)注射到体内, 阻抑肿瘤血管形成, 使胰腺癌细胞因供养不足而凋亡[63]; 也有利用免疫调控剂(如IL-2[64], virulizin等[65])或细胞凋亡抑制剂(如survivin[66])注射到体内, 以增强患者抗病能力, 延长存活期并改善生存质量; 此外, 亦有采用单株纯种抗体抑制胰腺癌中高度表达的结缔组织生长因子, 以期控制肿瘤的生长和转移[67]; 还有用基因治疗联合免疫治疗(如UPRT/5-FC联合TRA-8)以增强抗癌能力[68-69].
人类80%-90%的癌症与环境相关, 其中约35%与饮食有关, 饮食调理的目的, 是合理安排饮食, 保证充分的营养, 提高身体免疫功能, 巩固治疗效果, 防止癌症复发. 研究显示, 多吃蔬果(如卷心菜)可减低患胰腺癌的机会[70]. 而中医食疗则提倡和胃通腑、清肝消痞、健脾益气, 现列举一些调理胰腺癌食疗如下: (1)牛奶淮山糊. 具健脾补中、生津养胃功效, 用于不思饮食者; (2)山楂香橼煎. 具理气消食、利膈祛瘀功效, 用于腹痛呕吐纳呆者; (3)粉葛猪胰汤. 具生津润燥、补脾益肺功效, 用于腹胀口干、便燥纳差者; (4)夏枯草甜瓜猪胰汤. 具清热利尿、养肝除烦、生津止渴功效, 用于纳差、形体消瘦、左胁疼痛者; (5)猪肉猪胰煲魔芋. 具解毒散结、补脾润燥功效, 用于上腹胀满疼痛者; (6)桂花莲子粥. 具化痰散瘀、补益脾胃功效, 用于腹胀痛纳差者; (7)黄花木耳瘦肉汤. 具清肝养胃、祛瘀退黄功效, 用于消瘦乏力伴腹胀黄疸者; (8)蜗牛瘦肉煲鸡骨草. 具清热利湿、疏肝和脾功效, 用于体虚纳呆伴黄疸腹水者; (9)大蒜田七焖鳝鱼. 具补虚健脾、祛瘀止痛功效, 用于晚期腹胀疼痛、体虚纳差者; (10)桃仁人参粥. 具补中益气、润燥祛瘀功效, 用于晚期腹痛呕吐、形神俱衰者.
胰腺癌治愈率低, 生存期短, 患上本病几近被判死刑, 虽然外科手术、放疗和化疗技术日益进步, 但依然未能控制此顽疾. 治癌如用兵, 有正有奇, 正者法度, 奇者不为法度所绑. 中医辨证施治、标本兼治、阴阳平衡等方法和理论, 如能恰当地融合到西医对胰腺癌的综合诊疗中, 定将提高治愈率并能改善患者的生存质量. 另一方面, 癌基因的发现及其功能的逐步明确, 无疑是肿瘤学发展史上的重要里程碑, 胰腺癌研究得以进入一个崭新的分子时代. 分子靶向治疗在近年来蓬勃兴起, 尽管其技术仍需改善, 疗效有待提高, 但已给恶性肿瘤的诊疗带来了曙光, 不难预见, 将来胰腺癌治疗的突破很可能依赖个体化中西医综合治疗的实践和分子靶向治疗的成熟应用.
胰腺癌是死亡率最高的恶性肿瘤之一, 由于难以早期确诊, 增加了手术的局限性, 加上本病对放、化疗不敏感, 患者平均生存期少于6 mo, 近年个体化中西医综合诊疗的提倡, 与分子靶向治疗的迅猛发展, 为胰腺癌的治疗带来了新希望.
人类基因图谱的完成是恶性肿瘤研究的新的里程碑, 先进的基因组和蛋白质组科研平台, 加速了分子靶向诊疗的研究发展, 本文对前沿性的胰腺癌分子靶向治疗作了具体的阐述.
本文对胰腺癌的诊疗做了较全面的总结和资料汇编, 更针对胰腺癌的特性, 详细介绍其中西医综合诊疗的方法, 对易为人忽略的饮食调理和先进的分子靶向治疗亦有详尽的介绍, 对临床研究有一定参考作用.
甲基化特异性基因扩增(MSP): 用基因扩增技术直接检测基因组上癌基因或抑癌基因的甲基化状态, 显示基因是否活化, 来预示癌变的可能性, 如抑癌基因p16的GC岛在多种癌变中甲基化, 被抑制表达, 通过MSP可检测此基因GC岛的甲基化情况.
表面增强激光解吸离子化: 飞行时间质谱(SELDITOFMS)-利用激光脉冲使芯片中的分析物解吸形成荷电离子, 根据不同质荷比, 离子在仪器场中飞行的时间长短不一, 由此绘制出一张质谱图来, 经计算机处理可形成模拟谱图, 直接显示样品中各种蛋白质的分子量、含量等信息, 将它与常人或某种疾病患者谱图进行对照, 能够发现和捕获新的疾病特异性相关蛋白质及其特征.
小型干扰核糖核酸(siRNA): 是抑制目标基因的有力手段, 运用21-25个双链的siRNA来干扰目标基因表达, siRNA与目标基因是同源的, 而改良的叉状双链siRNA, 其有义链的3'端有1-4个与反义链不匹配的碱基, 这种分子在哺乳类动物细胞中能够增加传统RNAi的强度.
本文对胰腺癌的诊疗做出了全面的总结和资料汇编, 针对胰腺癌的特性, 详细介绍其中西医综合诊疗、饮食调理以及先进的分子靶向治疗, 对临床研究有一定参考作用.
电编: 李琪 编辑:潘伯荣
| 1. | Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108. [PubMed] [DOI] |
| 2. | Laheru D, Biedrzycki B, Thomas AM, Jaffee EM. Development of a cytokine-modified allogeneic whole cell pancreatic cancer vaccine. Methods Mol Med. 2005;103:299-327. [PubMed] |
| 3. | Gallicchio L, Kouzis A, Genkinger JM, Burke AE, Hoffman SC, Diener-West M, Helzlsouer KJ, Comstock GW, Alberg AJ. Active cigarette smoking, household passive smoke exposure, and the risk of developing pancreatic cancer. Prev Med. 2006;42:200-205. [PubMed] [DOI] |
| 4. | Malfertheiner P, Schutte K. Smoking-a trigger for chronic inflammation and cancer development in the pancreas. Am J Gastroenterol. 2006;101:160-162. [PubMed] [DOI] |
| 5. | Wang L, Li H. Advances in researches on genetic epidemiology of pancreatic cancer. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2006;28:289-293. [PubMed] |
| 6. | Valerio A, Basso D, Fogar P, Falconi M, Greco E, Bassi C, Seraglia R, Abu-Hilal M, Navaglia F, Zambon CF. Maldi-TOF analysis of portal sera of pancreatic cancer patients: identification of diabetogenic and antidiabetogenic peptides. Clin Chim Acta. 2004;343:119-127. [PubMed] [DOI] |
| 7. | Saftoiu A, Popescu C, Cazacu S, Dumitrescu D, Georgescu CV, Popescu M, Ciurea T, Gorunescu F. Power Doppler endoscopic ultrasonography for the differential diagnosis between pancreatic cancer and pseudotumoral chronic pancreatitis. J Ultrasound Med. 2006;25:363-372. [PubMed] |
| 8. | Koopmann J, Rosenzweig CN, Zhang Z, Canto MI, Brown DA, Hunter M, Yeo C, Chan DW, Breit SN, Goggins M. Serum markers in patients with resectable pancreatic adenocarcinoma: macrophage inhibitory cytokine 1 versus CA19-9. Clin Cancer Res. 2006;12:442-446. [PubMed] [DOI] |
| 9. | Choudhuri G, Singh D. Molecular mechanisms of pancreatic cancer. Trop Gastroenterol. 2005;26:111-114. |
| 10. | He Y, Yang B, Ruan CG. Anti-pancreatic cancer immune response induced by K-ras mutated peptide. Ai Zheng. 2005;24:559-562. [PubMed] |
| 11. | Jeong S, Lee DH, Lee JI, Lee JW, Kwon KS, Kim PS, Kim HG, Shin YW, Kim YS, Kim YB. Expression of Ki-67, p53, and K-ras in chronic pancreatitis and pancreatic ductal adenocarcinoma. World J Gastroenterol. 2005;11:6765-6769. [PubMed] [DOI] |
| 12. | Juuti A, Louhimo J, Nordling S, Ristimaki A, Haglund C. Cyclooxygenase-2 expression correlates with poor prognosis in pancreatic cancer. J Clin Pathol. 2006;59:382-386. [PubMed] [DOI] |
| 13. | Xu L, Li YM, Yu CH, Li L, Liu YS, Zhang BF, Fang J, Zhou Q, Hu Y, Gao HJ. Expression of p53, p16 and COX-2 in pancreatic cancer with tissue microarray. Hepatobiliary Pancreat Dis Int. 2006;5:138-142. [PubMed] |
| 14. | Zhang L, Chenwei L, Mahmood R, van Golen K, Greenson J, Li G, D'Silva NJ, Li X, Burant CF, Logsdon CD. Identification of a putative tumor suppressor gene Rap1GAP in pancreatic cancer. Cancer Res. 2006;66:898-906. [PubMed] [DOI] |
| 15. | Moniaux N, Nemos C, Schmied BM, Chauhan SC, Deb S, Morikane K, Choudhury A, Vanlith M, Sutherlin M, Sikela JM, Hollingsworth MA, Batra SK. The human homologue of the RNA polymerase II-associated factor 1 (hPaf1), localized on the 19q13 amplicon, is associated with tumorigenesis. Oncogene. 2006;25:3247-3257. [PubMed] [DOI] |
| 16. | Ohuchida K, Mizumoto K, Ishikawa N, Fujii K, Konomi H, Nagai E, Yamaguchi K, Tsuneyoshi M, Tanaka M. The role of S100A6 in pancreatic cancer development and its clinical implication as a diagnostic marker and therapeutic target. Clin Cancer Res. 2005;11:7785-7793. [PubMed] [DOI] |
| 17. | Li D, Frazier M, Evans DB, Hess KR, Crane CH, Jiao L, Abbruzzese JL. Single nucleotide polymorphisms of RecQ1, RAD54L, and ATM genes are associated with reduced survival of pancreatic cancer. J Clin Oncol. 2006;24:1720-1728. [PubMed] [DOI] |
| 18. | Matsubayashi H, Canto M, Sato N, Klein A, Abe T, Yamashita K, Yeo CJ, Kalloo A, Hruban R, Goggins M. DNA methylation alterations in the pancreatic juice of patients with suspected pancreatic disease. Cancer Res. 2006;66:1208-1217. [PubMed] [DOI] |
| 19. | Sato N, Fukushima N, Matsubayashi H, Iacobuzio-Donahue CA, Yeo CJ, Goggins M. Aberrant methylation of Reprimo correlates with genetic instability and predicts poor prognosis in pancreatic ductal adenocarcinoma. Cancer. 2006;. [PubMed] [DOI] |
| 20. | Smirne C, Camandona M, Alabiso O, Bellone G, Emanuelli G. High serum levels of Transforming Growth Factor-beta1, Interleukin-10 and Vascular Endothelial Growth Factor in pancreatic adenocarcinoma patients. Minerva Gastroenterol Dietol. 1999;45:21-27. [PubMed] |
| 21. | Wente MN, Jain A, Kono E, Berberat PO, Giese T, Reber HA, Friess H, Buchler MW, Reiter RE, Hines OJ. Prostate stem cell antigen is a putative target for immunotherapy in pancreatic cancer. Pancreas. 2005;31:119-125. [DOI] |
| 22. | Okada T, Akada M, Fujita T, Iwata T, Goto Y, Kido K, Okada T, Matsuzaki Y, Kobayashi K, Matsuno S. A novel cancer testis antigen that is frequently expressed in pancreatic, lung, and endometrial cancers. Clin Cancer Res. 2006;12:191-197. [DOI] |
| 23. | Mancuso A, Calabro F, Sternberg CN. Current therapies and advances in the treatment of pancreatic cancer. Crit Rev Oncol Hematol. 2006;58:231-241. [PubMed] [DOI] |
| 24. | Bloomston M, Bhardwaj A, Ellison EC, Frankel WL. Epidermal growth factor receptor expression in pancreatic carcinoma using tissue microarray technique. Dig Surg. 2006;23:74-79. [PubMed] [DOI] |
| 25. | Bloomston M, Zhou JX, Rosemurgy AS, Frankel W, Muro-Cacho CA, Yeatman TJ. Fibrinogen gamma overexpression in pancreatic cancer identified by large-scale proteomic analysis of serum samples. Cancer Res. 2006;66:2592-2599. [PubMed] [DOI] |
| 26. | Xia Q, Kong XT, Zhang GA, Hou XJ, Qiang H, Zhong RQ. Proteomics-based identification of DEAD-box protein 48 as a novel autoantigen, a prospective serum marker for pancreatic cancer. Biochem Biophys Res Commun. 2005;330:526-532. [PubMed] [DOI] |
| 27. | Yu KH, Rustgi AK, Blair IA. Characterization of proteins in human pancreatic cancer serum using differential gel electrophoresis and tandem mass spectrometry. J Proteome Res. 2005;4:1742-1751. [PubMed] [DOI] |
| 28. | Gronborg M, Kristiansen TZ, Iwahori A, Chang R, Reddy R, Sato N, Molina H, Jensen ON, Hruban RH, Goggins MG. Biomarker discovery from pancreatic cancer secretome using a differential proteomic approach. Mol Cell Proteomics. 2006;5:157-171. [PubMed] [DOI] |
| 29. | Chen R, Pan S, Yi EC, Donohoe S, Bronner MP, Potter JD, Goodlett DR, Aebersold R, Brentnall TA. Quantitative proteomic profiling of pancreatic cancer juice. Proteomics. 2006;. [PubMed] [DOI] |
| 30. | Rosty C, Christa L, Kuzdzal S, Baldwin WM, Zahurak ML, Carnot F, Chan DW, Canto M, Lillemoe KD, Cameron JL. Identification of hepatocarcinoma-intestine-pancreas/pancreatitis-associated protein I as a biomarker for pancreatic ductal adenocarcinoma by protein biochip technology. Cancer Res. 2002;62:1868-1875. [PubMed] |
| 31. | Honda K, Hayashida Y, Umaki T, Okusaka T, Kosuge T, Kikuchi S, Endo M, Tsuchida A, Aoki T, Itoi T. Possible detection of pancreatic cancer by plasma protein profiling. Cancer Res. 2005;65:10613-10622. [PubMed] [DOI] |
| 32. | Yang GY, Wagner TD, Fuss M, Thomas CR Jr. Multimodality approaches for pancreatic cancer. CA Cancer J Clin. 2005;55:352-367. [PubMed] [DOI] |
| 33. | Mann O, Strate T, Schneider C, Yekebas EF, Izbicki JR. Surgery for advanced and metastatic pancreatic cancer-current state and perspectives. Anticancer Res. 2006;26:681-686. [PubMed] |
| 34. | Fernandez JA, Parrilla P. What are the main errors made by surgeons in the management of pancreatic cancer? Cir Esp. 2006;79:215-223. [PubMed] |
| 35. | Abrams RA. Radiotherapy in the adjuvant management of pancreatic adenocarcinoma. Semin Oncol. 2005;32:S30-S32. [PubMed] [DOI] |
| 36. | Evans DB. Preoperative chemoradiation for pancreatic cancer. Semin Oncol. 2005;32:S25-S29. [PubMed] [DOI] |
| 37. | Knaebel HP, Marten A, Schmidt J, Hoffmann K, Seiler C, Lindel K, Schmitz-Winnenthal H, Fritz S, Herrmann T, Goldschmidt H. Phase III trial of postoperative cisplatin, interferon alpha-2b, and 5-FU combined with external radiation treatment versus 5-FU alone for patients with resected pancreatic adenocarcinoma-CapRI: study protocol [ISRCTN62866759]. BMC Cancer. 2005;5:37. [PubMed] [DOI] |
| 38. | Reni M, Pasetto L, Aprile G, Cordio S, Bonetto E, Dell'Oro S, Passoni P, Piemonti L, Fugazza C, Luppi G. Raltitrexed-eloxatin salvage chemotherapy in gemcitabine-resistant metastatic pancreatic cancer. Br J Cancer. 2006;94:785-791. [PubMed] [DOI] |
| 39. | Piacentini P, Donadelli M, Costanzo C, Moore PS, Palmieri M, Scarpa A. Trichostatin A enhances the response of chemotherapeutic agents in inhibiting pancreatic cancer cell proliferation. Virchows Arch. 2006;448:797-804. [PubMed] [DOI] |
| 40. | Tang PA, Tsao MS, Moore MJ. A review of erlotinib and its clinical use. Expert Opin Pharmacother. 2006;7:177-193. [PubMed] [DOI] |
| 41. | Eickhoff A, Martin W, Hartmann D, Eickhoff JC, Mohler M, Galle PR, Riemann JF, Jakobs R. A phase I/II multicentric trial of gemcitabine and epirubicin in patients with advanced pancreatic carcinoma. Br J Cancer. 2006;94:1572-1574. [PubMed] [DOI] |
| 42. | MacKenzie MJ. Molecular therapy in pancreatic adenocarcinoma. Lancet Oncol. 2004;5:541-549. [PubMed] [DOI] |
| 43. | Kindler HL. Front-line therapy of advanced pancreatic cancer. Semin Oncol. 2005;32:S33-S36. [PubMed] [DOI] |
| 44. | Bhattacharyya M, Lemoine NR. Gene therapy developments for pancreatic cancer. Best Pract Res Clin Gastroenterol. 2006;20:285-298. [PubMed] [DOI] |
| 45. | Yin S, Goodrich DW. Combination gene therapy with p53 and Thoc1/p84 is more effective than either single agent in an animal model of human pancreatic adenocarcinoma. Int J Oncol. 2006;28:781-785. [PubMed] [DOI] |
| 46. | Eisenberg DP, Adusumilli PS, Hendershott KJ, Yu Z, Mullerad M, Chan MK, Chou TC, Fong Y. 5-fluorouracil and gemcitabine potentiate the efficacy of oncolytic herpes viral gene therapy in the treatment of pancreatic cancer. J Gastrointest Surg. 2005;9:1068-1077. [PubMed] [DOI] |
| 47. | Cascante A, Huch M, Rodriguez LG, Gonzalez JR, Costantini L, Fillat C. Tat8-TK/GCV suicide gene therapy induces pancreatic tumor regression in vivo. Hum Gene Ther. 2005;16:1377-1388. [PubMed] [DOI] |
| 48. | Lebedeva IV, Sarkar D, Su ZZ, Gopalkrishnan RV, Athar M, Randolph A, Valerie K, Dent P, Fisher PB. Molecular target-based therapy of pancreatic cancer. Cancer Res. 2006;66:2403-2413. [PubMed] [DOI] |
| 49. | Giroux V, Malicet C, Barthet M, Gironella M, Archange C, Dagorn JC, Vasseur S, Iovanna JL. p8 is a new target of gemcitabine in pancreatic cancer cells. Clin Cancer Res. 2006;12:235-241. [PubMed] [DOI] |
| 50. | Dykxhoorn DM, Lieberman J. Running interference: prospects and obstacles to using small interfering RNAs as small molecule drugs. Annu Rev Biomed Eng. 2006;. [PubMed] [DOI] |
| 51. | Bhattacharyya M, Lemoine NR. Gene therapy developments for pancreatic cancer. Best Pract Res Clin Gastroenterol. 2006;20:285-298. [PubMed] [DOI] |
| 52. | Tsutsumida H, Swanson BJ, Singh PK, Caffrey TC, Kitajima S, Goto M, Yonezawa S, Hollingsworth MA. RNA interference suppression of MUC1 reduces the growth rate and metastatic phenotype of human pancreatic cancer cells. Clin Cancer Res. 2006;12:2976-2987. [PubMed] [DOI] |
| 53. | Schneider G, Weber A, Zechner U, Oswald F, Friess HM, Schmid RM, Liptay S. GADD45alpha is highly expressed in pancreatic ductal adenocarcinoma cells and required for tumor cell viability. Int J Cancer. 2006;118:2405-2411. [PubMed] [DOI] |
| 54. | Deng X, Ewton DZ, Li S, Naqvi A, Mercer SE, Landas S, Friedman E. The kinase Mirk/Dyrk1B mediates cell survival in pancreatic ductal adenocarcinoma. Cancer Res. 2006;66:4149-4158. [PubMed] [DOI] |
| 55. | Li Y, Jian Z, Xia K, Li X, Lv X, Pei H, Chen Z, Li J. XIAP is related to the chemoresistance and inhibited its expression by RNA interference sensitize pancreatic carcinoma cells to chemotherapeutics. Pancreas. 2006;32:288-296. [PubMed] [DOI] |
| 56. | Wang Z, Banerjee S, Li Y, Rahman KM, Zhang Y, Sarkar FH. Down-regulation of notch-1 inhibits invasion by inactivation of nuclear factor-kappaB, vascular endothelial growth factor, and matrix metalloproteinase-9 in pancreatic cancer cells. Cancer Res. 2006;66:2778-2784. [PubMed] [DOI] |
| 57. | Tsuji N, Asanuma K, Kobayashi D, Yagihashi A, Watanabe N. Introduction of a survivin gene-specific small inhibitory RNA inhibits growth of pancreatic cancer cells. Anticancer Res. 2005;25:3967-3972. [PubMed] |
| 58. | Guan HT, Xue XH, Dai ZJ, Wang XJ, Li A, Qin ZY. Down-regulation of survivin expression by small interfering RNA induces pancreatic cancer cell apoptosis and enhances its radiosensitivity. World J Gastroenterol. 2006;12:2901-2907. [PubMed] [DOI] |
| 59. | Guan HT, Xue XH, Wang XJ, Li A, Qin ZY. siRNA targeted against survivin induces apoptosis of pancreatic cancer cells. Nan Fang Yi Ke Da Xue Xue Bao. 2006;26:169-173. [PubMed] |
| 60. | Salmon JS, Lockhart AC, Berlin J. Anti-angiogenic treatment of gastrointestinal malignancies. Cancer Invest. 2005;23:712-726. [PubMed] [DOI] |
| 61. | Crane CH, Ellis LM, Abbruzzese JL, Amos C, Xiong HQ, Ho L, Evans DB, Tamm EP, Ng C, Pisters PW. Phase I trial evaluating the safety of bevacizumab with concurrent radiotherapy and capecitabine in locally advanced pancreatic cancer. J Clin Oncol. 2006;24:1145-1151. [PubMed] [DOI] |
| 62. | Hase R, Miyamoto M, Uehara H, Kadoya M, Ebihara Y, Murakami Y, Takahashi R, Mega S, Li L, Shichinohe T, Kawarada Y, Kondo S. Pigment epithelium-derived factor gene therapy inhibits human pancreatic cancer in mice. Clin Cancer Res. 2005;11:8737-8744. [PubMed] [DOI] |
| 63. | Xie K, Wei D, Huang S. Transcriptional anti-angiogenesis therapy of human pancreatic cancer. Cytokine Growth Factor Rev. 2006;17:147-156. [PubMed] [DOI] |
| 64. | Angelini C, Bovo G, Muselli P, Mussi C, Crippa S, Caprotti R, Uggeri F. Preoperative interleukin-2 immunotherapy in pancreatic cancer: preliminary results. Hepatogastroenterology. 2006;53:141-144. [PubMed] |
| 65. | Li H, Cao MY, Lee Y, Lee V, Feng N, Benatar T, Jin H, Wang M, Der S, Wright JA. Virulizin, a novel immunotherapy agent, activates NK cells through induction of IL-12 expression in macrophages. Cancer Immunol Immunother. 2005;54:1115-1126. [PubMed] [DOI] |
| 66. | Wobser M, Keikavoussi P, Kunzmann V, Weininger M, Andersen MH, Becker JC. Complete remission of liver metastasis of pancreatic cancer under vaccination with a HLA-A2 restricted peptide derived from the universal tumor antigen survivin. Cancer Immunol Immunother. 2006;55:1294-1298. [PubMed] [DOI] |
| 67. | Dornhofer N, Spong S, Bennewith K, Salim A, Klaus S, Kambham N, Wong C, Kaper F, Sutphin P, Nacalumi R. Connective tissue growth factor-specific monoclonal antibody therapy inhibits pancreatic tumor growth and metastasis. Cancer Res. 2006;66:5816-5827. [PubMed] [DOI] |
| 68. | Plate JM, Harris JE. Immunobiotherapy directed against mutated and aberrantly expressed gene products in pancreas cancer. J Cell Biochem. 2005;94:1069-1077. [PubMed] |
| 69. | Kaliberov SA, Chiz S, Kaliberova LN, Krendelchtchikova V, Della Manna D, Zhou T, Buchsbaum DJ. Combination of cytosine deaminase suicide gene expression with DR5 antibody treatment increases cancer cell cytotoxicity. Cancer Gene Ther. 2006;13:203-214. [PubMed] [DOI] |
| 70. | Larsson SC, Hakansson N, Naslund I, Bergkvist L, Wolk A. Fruit and vegetable consumption in relation to pancreatic cancer risk: a prospective study. Cancer Epidemiol Biomarkers Prev. 2006;15:301-305. [PubMed] [DOI] |