修回日期: 2006-03-27
接受日期: 2006-04-06
在线出版日期: 2006-06-08
目的: 探讨甘氨酸(Gly)对内毒素(LPS)性肝损害的保护机制.
方法: BALB/c小鼠随机分为三组, LPS组(n = 50)经腹腔注射10 mg/kg的LPS, Gly组(n = 50)在注射相同剂量LPS前3 d开始喂饲含50 g/L的Gly的饲料, 正常生理盐水对照组(n = 50), 经腹腔注射等体积的生理盐水, 光镜观察组织病理学改变, 免疫组织化学法检测TLR4表达水平; ELISA法检测血浆TNF-α, IL-10浓度及RT-PCR检测肝组织中TNF-α, IL-10及TLR4的mRNA表达水平.
结果: Gly能明显提高小鼠存活率, 肝脏病理损害程度减轻; Gly组TNF-α水平显著低于LPS组, 差异有统计学意义(708.83±51.29 ng/L vs 1852.8±126.64 ng/L, F = 786.21, P<0.05); Gly组IL-10增加且高峰前移, 与LPS组比较差异有统计学意义(418.64±38.86 ng/L vs 211.15±26.44 ng/L, P<0.05); Gly组肝组织中TNF-α及TLR4表达也明显减弱, IL-10表达明显增强, 与LPS组比较差异均有统计学意义(分别为TNF-αA值: 1.59±0.14 vs 0.91±0.11; TLR4 A值: 0.97±0.12 vs 0.53±0.11; IL-10 A值: 0.62±0.08 vs 1.06±0.15;P均<0.05).
结论: Gly能明显减轻LPS所致的肝损害, 其机制可能与其下调肝细胞的TLR4表达, 同时上调IL-10的水平有关.
引文著录: 王强, 张涛, 陈叶恒, 余和斌, 游海波. 甘氨酸对内毒素性肝损害保护作用的机制. 世界华人消化杂志 2006; 14(16): 1561-1565
Revised: March 27, 2006
Accepted: April 6, 2006
Published online: June 8, 2006
AIM: To explore the protective mechanisms of glycine on liopolysaccharide (LPS)-induced liver injury.
METHODS: BABL/c mice were randomly divided into LPS group (n = 50), glycine group (n = 50), and control group (n = 50). The animals in LPS and glycine groups were intraperitoneally injected with 10 mg/kg LPS to establish the liver injury model, while animals in glycine group were pretreated with 50 g/L glycine-containing food 3 d before modeling. The mice in control group received the same amount of normal saline. Liver samples were collected to observe histopathological changes. The levels of tumor necrosis factor-a (TNF-α) and interleukin-10 (IL-10) in plasma were measured by enzyme linked immunosorbent assay (ELISA). The mRNA expression of TNF-α, IL-10 and toll like receptor 4 (TLR4) in hepatic tissues were detected by reverse transcription-polymerase chain reaction (RT-PCR). The expression of TLR4 protein in liver was detected with immunohistochemistry.
RESULTS: Glycine improved the survival rates of the mice, and attenuated LPS-induced pathological changes of the liver tissues significantly. The plasma level of TNF-α was markedly lower in glycine group than that in LPS group (708.83 ± 51.29 ng/L vs1852.8 ± 126.64 ng/L, F = 786.21, P < 0.05) at 3 h, while the level of IL-10 was significantly higher at 12 h (418.64 ± 38.86 ng/L vs 211.15 ± 26.44 ng/L, P < 0.05). The expression of TNF-α and TLR4 were notably decreased in glycine group in comparison with those in LPS group (A value: 1.59 ± 0.14 vs 0.91 ± 0.11, P < 0.05; 0.97 ± 0.12 vs 0.53 ± 0.11, P < 0.05), but the expression of IL-10 was remarkably increased (A value: 0.62 ± 0.08 vs 1.06 ± 0.15, P < 0.05).
CONCLUSION: Glycine pretreatment can attenuate LPS-induced liver injury, which may be associated with the down-regulation of TLR4 expression and up-regulation of IL-10 production.
- Citation: Wang Q, Zhang T, Chen YH, Yu HB, You HB. Protection mechanisms of glycine against liver injury induced by liopolysaccharide. Shijie Huaren Xiaohua Zazhi 2006; 14(16): 1561-1565
- URL: https://www.wjgnet.com/1009-3079/full/v14/i16/1561.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v14.i16.1561
脂多糖(lipopolysaccharide, LPS)是革兰阴性细菌细胞壁上的主要成分, 主要通过与单核-巨噬细胞表面的跨膜受体Toll样受体4(toll like receptor 4, TLR4)等相结合后, 引起肿瘤坏死因子-a(TNF-α)等大量促炎症介质释放, 导致全身性炎症反应及多器官功能损害[1-3]. 由于全身80%-90%的单核-巨噬细胞为位于肝血窦内库普弗细胞, 因此在内毒素血症时库普弗细胞及肝脏成为内毒素的主要靶细胞和器官[4-5]. 甘氨酸(glycine, Gly)能明显减轻内毒素性休克大鼠模型肝细胞坏死[6], 但其作用机制尚未明确. 本实验拟通过观察Gly预处理对LPS攻击小鼠肝脏的LPS受体TLR4及其下游细胞因子表达的影响, 探讨LPS对肝损害的机制及Gly减轻其肝损害的可能机制.
♀清洁级健康Balb/c小鼠, 体质量18±2 g, 10-12周龄(重庆医科大学动物实验中心); LPS、甘氨酸(Sigma); 大鼠抗小鼠TLR4IgG (Santa Cruze); AMV逆转录酶(TaKaRa); 小鼠TNF-α、IL-10 ELISA试剂盒(武汉博士德生物技术有限公司); 两步法RT-PCR试剂盒(大连宝生物工程有限公司); 凝胶成像分析系统(Bio-Rad Doc Gel 2000); 电泳仪(300 V, Bio-Rad); 全自动生化分析仪(BECKMAN CX7); 低温高速离心机(Mikro 22 R).
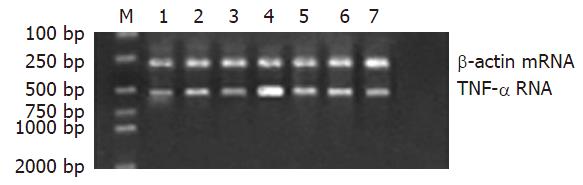
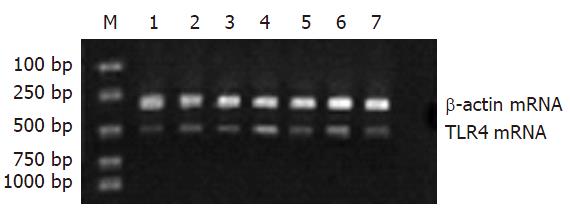
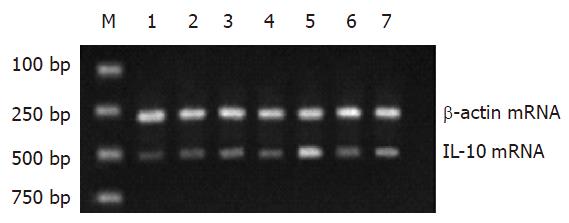
(1)BALB/c小鼠150只, 随机分为3组(n = 50). 内毒素组(LPS组), 禁食12 h后, 经腹腔注射10 mg/kg的LPS, 建立内毒素血症肝损害动物模型; 甘氨酸组(Gly组), 腹腔注入相同剂量的LPS前3 d, 喂饲含50 g/kg的Gly饲料; 正常生理盐水对照组(n = 50), 经腹腔注射等体积的生理盐水, 每组各取20只小鼠, LPS处理后观察5 d, 记录存活率; 剩余动物于注射LPS溶液后0, 1, 3, 6, 12 h各时相点活杀取材(每组每时点各6只). (2)光镜检查: 取左肝外叶组织, 常规石蜡包埋、HE染色. (3)免疫组织化学染色: 取左肝外叶组织, 采用SABC法, 按试剂盒说明书检测TLR4蛋白的表达. (4)血浆ALT浓度测定: 抽取肝下腔静脉血液3 mL, 用全自动血液分析仪直接测定ALT. (5)血浆中TNF-α、IL-10含量测定: 采用ELISA法, 按相应的试剂盒说明书进行. (6)肝组织TNF-α、IL-10、TLR4 mRNA表达水平检测: 采用RT-PCR法. PCR引物由上海鼎安生物科技有限公司设计并合成, TLR4, 上游引物5'-CAAGGCATGGCATGGCTTAC-3', 下游引物3'-GGTAGCCAACTAGAACCCTC-5', 扩增产物500 bp; TNF-α, 上游引物5'-TCTACTGAACTTCGGGGTGA-3', 下游引物3'-CTCAGGCCCGT CCAGA TGA-5', 扩增产物542 bp; IL-10, 上游引物5'-CCAAGCTTATCGGAAATG-3', 下游引物3'-CACTTGTA AATCTTTCTTCGGG-5', 扩增产物500503 bp; 内参照b-actin, 上游引物5'-CATTGTGATGGAC TCCGGAG-3', 下游引物3'-CTGCCGGTCCAGTAGTGATA-5', 扩增产物300 bp; 扩增产物电泳结果采用Doc Gel 2000凝胶图像分析系统成像, 并以Bio-Image Analysis System进行半定量分析, 结果以相对吸光度值A×面积(mm2)表示.
统计学处理 采用SPSS 10.0软件进行, 组间均数的两两比较用t检验, 组内均数的两两比较用q检验, 组间率的两两比较用χ2检验, 以P<0.05为差异有显著性.
观察期内, LPS组和Gly组小鼠24, 48, 72, 120 h的存活率分别为50.0%, 40.0%, 30.0%, 30.0%和90.0%, 80.0%, 70.0%, 70.0%, 两者比较差异显著(P<0.05).
光镜结果显示: LPS组3 h时, 肝细胞出现肿胀、空泡样变性、汇管区有炎性细胞浸润, 随时间的延长, 上述损害加重, 12 h时除上述损害外可见中央静脉内有血栓形成, 24 h时可见点片状、大片状甚至肝窦结构被破坏; Gly组, 形态学上肝细胞肿胀、空泡样变性明显减轻, 汇管区炎性细胞浸润减少, 整个观察时间内仅见点片状坏死, 未见大片状及肝窦结构的破坏.
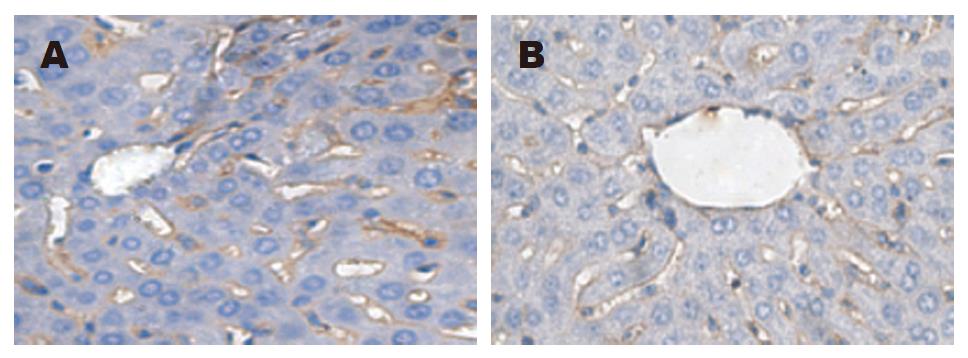
免疫组化结果显示: LPS组3 h时肝细胞、血管内皮细胞内可见大量棕黄色颗粒, 且主要分布于肝血窦周围. Gly组3 h时阳性反应细胞也主要分布于肝血窦周围, 但比LPS组数目少且染色程度轻.
Gly组相同时相点的ALT含量明显低于LPS组, 差异有统计学意义(F = 558.63, P<0.05)(表1).
Gly组TNF-α的水平, 明显低于同时点的LPS组, 差异有统计学意义(F = 786.21, P<0.05)(表2). Gly预处理组血浆IL-10含量在LPS刺激1 h后即开始明显增加, 且高峰提前至12 h, 和同时点的LPS组, 差异有统计学意义(F = 311.12, P<0.05)(表3).
RT-PCR结果显示: Gly预处理TLR4、TNF-α mRNA水平明显低于同时点的LPS组, 差异具显著性(P<0.05); IL-10 mRNA水平明显高于同时点的LPS组, 差异具显著性(P<0.05).
内毒素血症至今仍是临床治疗中的难点, 现行的抗感染治疗方案中, 主要原则是及早全身使用抗生素, 但其直接后果却是在迅速杀死细菌的同时导致短时间内大量的LPS释放入血, 可能加重患者的临床毒血症反应, 因此, 严重感染后脓毒性休克病死率至今仍高达70%[7-8]. 学者们一直在寻找较为理想的LPS拮抗剂, 但多因拮抗效果差或不良反应大等而不能用于临床[9]. 因此, 我们拟通过建立内毒素性肝损害动物模型, 观察Gly对肝脏的保护效应, 并探讨保护机制, 试图为内毒素性肝损害的临床治疗提供新的手段.
LPS活化细胞是通过一系列信号转导事件实现的[10-13]. TLR4是负责LPS信号跨膜转导的主要LPS受体, 也是LPS向细胞内传递信号的必经门户. 研究表明C3H/HeJ小鼠TLR4基因q32-33处的点突变, 导致其编码的蛋白质多肽第712位的脯氨酸被组氨酸取代, 或C57BL/10ScCr小鼠系的TLR4基因的完全缺乏, 从而改变了TLR4的信号转导功能, 均导致两者对LPS的高度耐受, 因此TLR4被认为是LPS信号传递的关键蛋白[14-15].
动物实验表明, 随时间推移, 两组小鼠肝脏病变、血浆ALT的含量均进行性加重(增高), 但由于Gly组的TNF-α浓度明显低于LPS组, 因此, 其肝损害程度及死亡率也明显低于后者. TLR4免疫组织化学染色显示, 在肝细胞、窦内皮细胞、库普弗细胞可见棕黄色颗粒, 这提示肝细胞、窦内皮细胞同库普弗细胞一样, 也存在TLR4, 由于阳性颗粒主要分布于肝血窦周围, 说明TLR4主要在库普弗细胞中表达, 这与Ogushi et al[16]的研究结果一致. 肝组织TLR4 mRNA的表达变化趋势与TNF-α mRNA、血浆TNF-α水平一致, 这提示血浆TNF-α水平与肝脏细胞TLR4 mRNA表达有关. 故我们认为LPS致肝损害的主要机制为: 由于肝脏细胞受到LPS信号刺激后, 其膜上的LPS识别受体TLR4表达迅速增强, 通过上述机制造成肝脏损害. 由于TLR4阳性反应细胞主要为库普弗细胞, 全身80%-90%的单核-巨噬细胞为位于肝血窦内的库普弗细胞, 其过度激活所产生的大量炎性介质, 必将造成肝损害. 因而在内毒素致肝损害中, 库普弗细胞的过度激活在其中起着比肝细胞、窦内皮细胞更为重要的作用. 因此, 目前针对TLR为靶点的药物研究也正成为一个热点, 并有可能为提高脓毒性休克及其他感染性疾病的疗效带来希望. 以上的研究结果提示我们, 通过干预、调节、抑制TLR4的表达可望成为一种治疗脓毒症新的手段.
Gly作为机体的一种非必需氨基酸, 可以在细胞、器官、整体水平发挥机体的保护作用; 将其应用于酒精、药物性(环抱霉素A、D-Gal)肝损害、肝脏/缺血再灌注模型以及肝移植模型等, 均显示出良好的保护作用[17-21], 但其作用机制仍不十分清楚. 孙葳 et al[22]研究认为Gly可能是通过带正电荷的NH4+与血浆中LPS脂质A上带负电荷的PO43-结合, 破坏了LPS的结构, 并在一定程度上干扰了巨噬细胞的结合. 也有研究认为库普弗细胞膜上存在Gly受体, Gly通过与其受体结合后, 大量氯离子内流使细胞膜超级化, 钙离子内流减少, 使钙依赖的激酶不能被激活, 从而下调TNF-α水平, 使其后续事件不能发生[23-26]. 动物实验表明Gly的这种保护作用似与其独特的分子构型有关, 原因如下: (1)动物实验表明Gly能增加肝组织IL-10的表达, IL-10是体内天然存在的抑制炎症, 维持细胞因子网络平衡的小分子. 通过抑制单核-巨噬细胞主要组织相容性复合物Ⅱ类抗原的表达, 既抑制Th-1细胞和单核细胞活化而减少炎性介质TNF-α等的生成, 又对LPS诱导的一氧化氮、氧自由基、血小板活化因子的休克介质有下调作用[27-30]. (2)Gly作用后, 通过某种受体, 下调肝脏各种细胞中(特别是库普弗细胞)TLR4的表达, 抑制LPS与TLR4的结合, 发挥LPS受体类似拮抗剂的作用, 最终通过抑制TNF-α表达和产生, 从而明显减轻了内毒素的损伤效应.
内毒素血症至今仍是临床治疗中的难点, 现行的抗感染治疗方案中, 主要原则是及早全身使用抗生素, 但其直接后果却是在迅速杀死细菌的同时导致短时间内大量的内毒素释放入血, 可能加重患者的临床毒血症反应, 因此, 严重感染后脓毒性休克病死率至今仍高达70%以上. 学者们一直在寻找较为理想的内毒素拮抗剂, 但多因拮抗效果差, 或本身的毒副作用大等而不能用于临床.
大量研究新表明甘氨酸(glycine, Gly)对缺血-再灌注、缺氧等损害均显示出保护作用, 是一种新发现的细胞保护剂. 因此, 本文拟通过建立内毒素性肝损害动物模型, 观察Gly对肝脏的保护效应, 探讨保护机制, 并试图为内毒素性肝损害的临床治疗提供新的手段. 因为Gly为机体的一种非必需氨基酸, 其毒副作用小, 可以进一步经临床试验验证后, 用于临床内毒素血症的防治.
Gly为机体的一种非必需氨基酸, 其毒副作用小, 进一步经临床试验验证后, 可以用于临床内毒素血症的防治, 其机制可能与其下调肝脏各种细胞的TLR4表达, 同时上调抗炎因子IL-10的水平有关.
本文从LPS信号转导角度, 研究发现Gly作用后, 通过下调肝脏细胞中TLR4的表达, 抑制LPS与TLR4的结合, 发挥LPS受体类似拮抗剂的作用, 最终通过抑制TNF-α表达, 而明显减轻了内毒素的损伤效应. 研究具有一定的探索性和创新性.
电编: 张敏 编辑:潘伯荣
| 1. | Giamarellos-Bourboulis EJ, Baziaka F, Antonopou-lou A, Koutoukas P, Kousoulas V, Sabracos L, Panagou C, Perrea D, Giamarellou H. Clarithromy-cin co-administered with amikacin attenuates systemic inflammation in experimental sepsis with Escherichia coli. Int J Antimicrob Agents. 2005;25:168-172. [PubMed] [DOI] |
| 2. | Volman TJ, Goris RJ, van der Meer JW, Hendriks T. Tissue- and time-dependent upregulation of cytokine mRNA in a murine model for the multiple organ dysfunction syndrome. Ann Surg. 2004;240:142-150. [PubMed] [DOI] |
| 3. | Fukui M, Imamura R, Umemura M, Kawabe T, Suda T. Pathogen-associated molecular patterns sensitize macrophages to Fas ligand-induced apoptosis and IL-1 beta release. J Immunol. 2003;171:1868-1874. [PubMed] [DOI] |
| 4. | Liu S, Gallo DJ, Green AM, Williams DL, Gong X, Shapiro RA, Gambotto AA, Humphris EL, Vodovotz Y, Billiar TR. Role of toll-like receptors in changes in gene expression and NF-kappa B activation in mouse hepatocytes stimulated with lipopolysaccharide. Infect Immun. 2002;70:3433-3442. [PubMed] [DOI] |
| 5. | Monshouwer M, Hoebe KH. Hepatic (dys-)function during inflammation. Toxicol In Vitro. 2003;17:681-686. [PubMed] [DOI] |
| 6. | Zhong Z, Wheeler MD, Li X, Froh M, Schemmer P, Yin M, Bunzendaul H, Bradford B, Lemasters JJ. L-Glycine: a novel antiinflammatory, immunomo-dulatory, and cytoprotective agent. Curr Opin Clin Nutr Metab Care. 2003;6:229-240. [PubMed] [DOI] |
| 7. | Vincent JL, Abraham E, Annane D, Bernard G, Rivers E, Van den Berghe G. Reducing mortality in sepsis: new directions. Crit Care. 2002;6 Suppl 3:S1-18. [PubMed] [DOI] |
| 8. | Moynagh PN. Toll-like receptor signalling pathways as key targets for mediating the anti-inflammatory and immunosuppressive effects of glucocorticoids. J Endocrinol. 2003;179:139-144. [PubMed] [DOI] |
| 9. | Yang S, Koo DJ, Chaudry IH, Wang P. Glycine attenuates hepatocellular depression during early sepsis and reduces sepsis-induced mortality. Crit Care Med. 2001;29:1201-1206. [PubMed] [DOI] |
| 10. | Yamamoto M, Takeda K, Akira S. TIR domain-containing adaptors define the specificity of TLR signaling. Mol Immunol. 2004;40:861-868. [PubMed] [DOI] |
| 11. | Guha M, Mackman N. LPS induction of gene expre-ssion in human monocytes. Cell Signal. 2001;13:85-94. [PubMed] [DOI] |
| 12. | Takeda K, Akira S. Toll-like receptors in innate immunity. Int Immunol. 2005;17:1-14. [PubMed] [DOI] |
| 13. | Brown V, Brown RA, Ozinsky A, Hesselberth JR, Fields S. Binding specificity of Toll-like receptor cytoplas-mic domains. Eur J Immunol. 2006;36:742-753. [PubMed] [DOI] |
| 14. | Heumann D, Roger T. Initial responses to endotoxins and Gram-negative bacteria. Clin Chim Acta. 2002;323:59-72. [PubMed] [DOI] |
| 15. | Iwami KI, Matsuguchi T, Masuda A, Kikuchi T, Musikacharoen T, Yoshikai Y. Cutting edge: naturally occurring soluble form of mouse Toll-like receptor 4 inhibits lipopolysaccharide signaling. J Immunol. 2000;165:6682-6686. [PubMed] [DOI] |
| 16. | Ogushi I, Iimuro Y, Seki E, Son G, Hirano T, Hada T, Tsutsui H, Nakanishi K, Morishita R, Kaneda Y. Nuclear factor kappa B decoy oligode-oxynucleotides prevent endotoxin-induced fatal liver failure in a murine model. Hepatology. 2003;38:335-344. [PubMed] [DOI] |
| 17. | Zhang SJ, Shi JH, Tang Z, Wu Y, Chen S. Protective effects of glycine pretreatment on brain-death do-nor liver. Hepatobiliary Pancreat Dis Int. 2005;4:37-40. [PubMed] |
| 18. | Peng Y, Gong JP, Liu CA, Li SW, Gan L, Li SB. The effect of glycine on CD14 and NF-kappa B in Kupffer cells from rat liver grafts after ischemia-reperfusion injury. Zhonghua Ganzangbing Zazhi. 2005;13:179-182. [PubMed] |
| 19. | Bruck R, Wardi J, Aeed H, Avni Y, Shirin H, Avinoach I, Shahmurov M, Hershkoviz R. Glycine modulates cytokine secretion, inhibits hepatic damage and improves survival in a model of endot-oxemia in mice. Liver Int. 2003;23:276-282. [PubMed] [DOI] |
| 20. | Yamashina S, Ikejima K, Enomoto N, Takei Y, Sato N. Glycine as a therapeutic immuno-nutrient for alcoholic liver disease. Alcohol Clin Exp Res. 2005;29:162S-165S. [PubMed] [DOI] |
| 21. | Matilla B, Mauriz JL, Culebras JM, Gonzalez-Gallego J, Gonzalez P. Glycine: a cell-protecting anti-oxidant nutrient. Nutr Hosp. 2002;17:2-9. [PubMed] |
| 23. | Liu ZJ, You HB, Li XH, Chen XF, Liu HZ, Peng Y, Liu CA, Gong JP. The mechanism and treatment phases chosen of glycine for inhibition lipopolysa-charide induced Kupffer cells activation. Zhonghua Waike Zazhi. 2006;44:189-192. [PubMed] |
| 24. | Qu W, Ikejima K, Zhong Z, Waalkes MP, Thurman RG. Glycine blocks the increase in intracellular free Ca2+ due to vasoactive mediators in hepatic paren-chymal cells. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1249-1256. [PubMed] [DOI] |
| 25. | Froh M, Thurman RG, Wheeler MD. Molecular evidence for a glycine-gated chloride channel in macrophages and leukocytes. Am J Physiol Gastroin-test Liver Physiol. 2002;283:G856-863. [PubMed] [DOI] |
| 26. | Wheeler M, Stachlewitz RF, Yamashina S, Ikejima K, Morrow AL, Thurman RG. Glycine-gated chloride channels in neutrophils attenuate calcium influx and superoxide production. FASEB J. 2000;14:476-484. [PubMed] |
| 27. | Bruck R, Wardi J, Aeed H, Avni Y, Shirin H, Avinoach I, Shahmurov M, Hershkoviz R. Glycine modulates cytokine secretion, inhibits hepatic damage and improves survival in a model of endot-oxemia in mice. Liver Int. 2003;23:276-282. [PubMed] [DOI] |
| 28. | Murphey ED, Traber DL. Protective effect of tumor necrosis factor-alpha against subsequent endotoxemia in mice is mediated, in part, by interleukin-10. Crit Care Med. 2001;29:1761-1766. [PubMed] [DOI] |