修回日期: 2006-03-10
接受日期: 2006-03-21
在线出版日期: 2006-05-08
目的: 调查蒙、汉族人群小肠脂肪酸结合蛋白(IFABP)基因exonⅡ54位点编码丙氨酸或苏氨酸(A/T)单核苷酸多态性(SNP), 探讨不同种族、饮食习惯与IFABP基因多态性频率分布的关系.
方法: 采用聚合酶链反应(PCR), DNA限制性内切酶酶切及基因测序等技术, 分别对208例牧区蒙古族人群、150例张家口市区蒙古族人群和190例汉族人群54A/T IFABP基因型分析.
结果: 牧区蒙古人群54T等位基因频率为0.51, 54A等位基因频率为0.49; 市区蒙古族人群54T等位基因频率为0.33, 54A等位基因频率为0.67; 汉族人群54T等位基因频率为0.30, 54A等位基因频率为0.70. 与市区蒙古族人群、汉族人群相比, 牧区蒙古族人群突变型54T等位基因频率明显增高, 且差别有统计学意义(分别为χ2 = 22.98, P<0.01; χ2 = 34.23, P<0.01). 市区蒙古族和汉族相比较, 突变型54T等位基因频率无明显差异(χ2 = 0.47, P>0.05).
结论: 蒙、汉族人群IFABP基因54A/T多态性频率分布无种族差别; 牧区蒙古族人群突变型54T IFABP基因高频率分布可能与高脂饮食习惯有关.
引文著录: 王振辉, 常晓彤, 侯小平, 董明纲, 王洪涛, 张利, 李桂喜. 蒙、汉族人群小肠脂肪酸结合蛋白基因Ala54Thr多态性的频率. 世界华人消化杂志 2006; 14(13): 1309-1313
Revised: March 10, 2006
Accepted: March 21, 2006
Published online: May 8, 2006
AIM: To investigate the single nucleotide polymorphism (SNP) at the 54Ala/Thr (A/T) in the intestinal fatty acid binding protein (IFABP) gene in Hans and Mongolians.
METHODS: Polymerase chain reaction (PCR), restriction endonuclease (HhaI) digestion and DNA sequencing technique were performed to detect the IFABP gene polymorphism at the 54Ala/Thr in 208 Mongolians of pastoral area, 150 Mongolians of Zhangjakou city and 190 Hans.
RESULTS: The allelic frequency of 54Thr was 0.51, 0.33, and 0.30, while that of 54Ala was 0.49, 0.67, and 0.70 in Mongolians of pastoral area, Zhangjakou city and 190 Hans, respectively. In comparison with that of Mongolians in urban area and Hans, the allelic frequency of codon 54Thr in Mongolians of pastoral area was significantly increased (χ2 = 22.98, P < 0.01; χ2 = 34.23, P < 0.01, respectively), however, it was not significantly different between the Mongolians of urban area and Hans.
CONCLUSION: The IFABP gene polymorphism at 54A/T has no correlation with ethnics among Mongolians and Hans, and the high frequency of 54Thr mutant genotype in Mongolians of pastoral area may be associated with high-fat dieting.
- Citation: Wang ZH, Chang XT, Hou XP, Dong MG, Wang HT, Zhang L, Li GX. Investigation of Ala54Thr polymorphism in intestinal fatty acid binding protein in Han and Mongoloid populations. Shijie Huaren Xiaohua Zazhi 2006; 14(13): 1309-1313
- URL: https://www.wjgnet.com/1009-3079/full/v14/i13/1309.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v14.i13.1309
基因多态性构成了不同个体与群体对疾病的易感性、对药物与环境因子不同反应的遗传学基础. 肥胖、高血脂、高血糖等代谢综合征的发生、发展受饮食、环境及遗传等多因素影响, 而胰岛素抵抗是其形成的中心环节. 在代谢综合征发生机制的探索中, 相关基因多态性改变越来越引起关注. IFABP定位于小肠上皮吸收细胞, IFABP基因exonⅡ54位点可编码丙氨酸或苏氨酸(A/T), 具有单核苷酸多态性. 近年来, 国外学者相继报道, 突变型54T IFABP与高脂血症、Ⅱ型糖尿病、胰岛素抵抗相关[1]. 我们通过对蒙、汉族人群IFABP基因多态性分析, 旨在探讨种族、饮食习惯与54A/T频率分布的关系.
研究对象: 张家口市区蒙古族人150例(市区居住3代以上, 饮食习惯与汉族类似), 男102例, 女48例, 平均年龄50.2±5.5岁; 牧区蒙古族人208例, 来自内蒙古集宁市中旗牧区, 男142例, 女66例, 平均年龄49.8±7.1岁; 张家口市汉族人190例, 男144例, 女46例, 平均年龄40.2±10.1岁. 以上研究对象均无血缘关系. 限制性内切酶HhaⅠ, Taq DNA聚合酶购自TaKaRa公司; 蛋白酶K购自Sigma公司; UNIQ-5胶回收试剂盒, PCR反应回收试剂盒购自上海生物工程有限公司. UNOⅡ型PCR仪(Biometra, 德国), UV-1601型紫外分光光度计, 75S/00771型凝胶成像分析系统(Bio Rad, 美国). 根据GenBank提供的人IFABP基因序列, 按引物设计原则, 将上游引物设计在距离突变位点200 bp, 下游引物设计在距离突变位点100 bp, 扩增产物长度为300 bp, 即上游引物序列: 5'-ACAGGTGTTAATATAGTGAAAAGG-3', 下游引物序列: 5'-ATTGGCTTCTTCAGTTAGTGAAGG-3'. GAPDH内参对照: 扩增片段长450 bp, 上游引物序列: 5'-ACCACAGTCCATGCCATCAC-3'; 下游引物序列: 5'-TCACCACCCTGTTGCTGTA-3'.
空腹静脉血1 mL, 38 g/L枸橼酸钠抗凝. 采用低渗溶血法分离白细胞; 酚/氯仿抽提法提取DNA. 目的IFABP基因片段扩增条件: 95℃预变性3 min; 94℃变性30 s, 56℃退火30 s, 72℃延伸1 min, 30个循环; 72℃补平8 min, 反应体系为50 μL. GAPDH内参扩增条件: 95℃预变性3 min; 94℃变性30 s, 55℃退火30 s, 72℃延伸1 min, 30个循环; 72℃补平8 min, 反应体系为25 μL. IFABP基因PCR扩增产物, 经PCR产物回收试剂盒回收后, 用限制性内切酶HhaⅠ, 37℃酶切过夜. 结果行30 g/L琼脂糖凝胶电泳, 经凝胶成像分析系统摄像, 并记录分析结果. 根据酶切结果, 针对性地选择部分完全切割、不完全切割和未切割的PCR产物, 经10 g/L琼脂糖凝胶电泳纯化回收, 待DNA序列分析比较, 测序分析由上海博亚生物技术有限公司完成.
统计学处理 基因型及等位基因频率采用基因计数法, 观察对象与Hardy-Weinberg平衡的符合程度、组间基因型及等位基因频率比较采用χ2检验. 以上统计均在SPSS 10.0软件上完成.
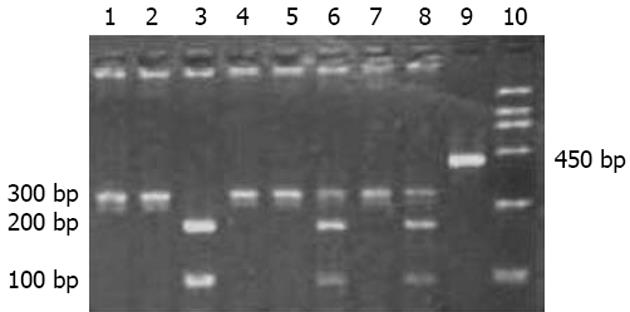
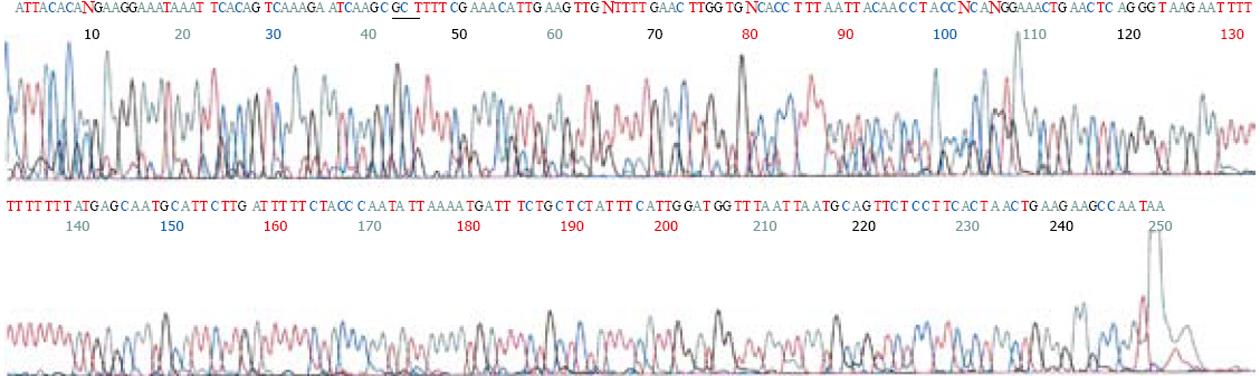
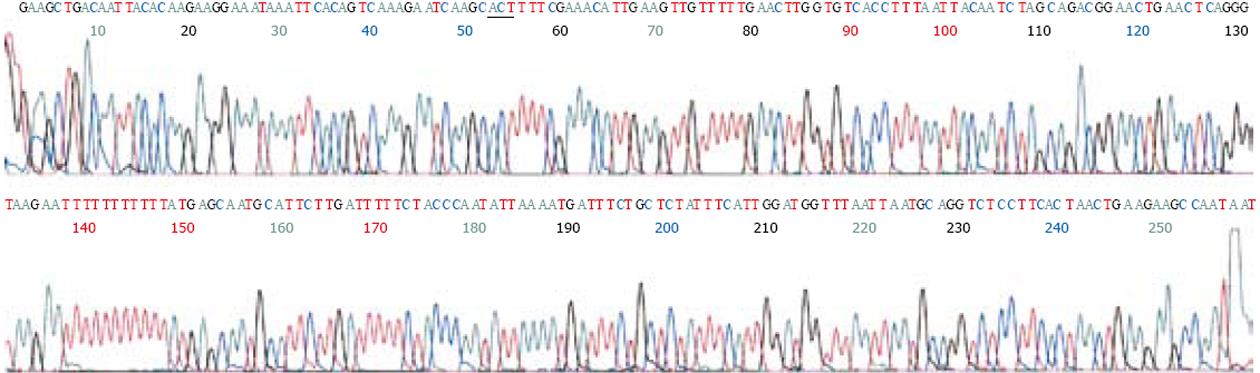
以外周血白细胞DNA为模板, 进行PCR扩增, 经10 g/L琼脂糖凝胶电泳观察, 在约300 bp处扩增出1条与预期值相符合的特异性IFABP基因片段, GAPDH内参扩增结果为450 bp. PCR产物经回收试剂盒回收后, 用限制性内切酶HhaⅠ, 37℃过夜酶切. HhaⅠ内切酶碱基识别序列为GCGC. 当IFABP基因54位点GCT(A)→ACT(T)时, 其所在碱基序列片段将由GCGCT→GCACT, HhaⅠ内切酶不能酶切. 酶切结果电泳分析显示3种情况: (1)突变纯合子型54T/T, 显示1条带, 分子量约为300 bp; (2)野生型54A/A, 显示2条带, 分子量约为200 bp和100 bp; (3)突变杂合子型54A/T, 显示3条带, 分子量分别约为300 bp, 200 bp和100 bp(图1). 图2所示为野生型54A/A(GCT/GCT)IFABP基因上游核苷酸序列片段测序结果; 图3所示为突变纯合子型54T/T(ACT/ACT)IFABP基因上游核苷酸序列片段测序结果.
150例市区蒙古族人群54A/T基因型分布: 野生型A/A 72例占0.48, 杂合子型A/T 57例占0.38, 突变纯合子型T/T 21例占0.14; 54T等位基因频率为0.33, 54A等位基因频率为0.67. 208例牧区蒙古族人群54A/T基因型分布: 野生型A/A 8例占0.04, 杂合子型A/T 198例占0.90, 突变纯合子型T/T 12例占0.06; 54T等位基因频率为0.51, 54A等位基因频率为0.49. 190例汉族人群54A/T基因型分布: 野生型A/A 96例占0.50, 杂合子型A/T 72例占0.38, 突变纯合子型T/T 22例占0.12; 54T等位基因频率为0.30, 54A等位基因频率为0.70. 与市区蒙古族人群相比, 牧区蒙古族人群54T等位基因频率明显增高, 且差别有统计学意义(χ2 = 22.98, P<0.01); 与汉族人群相比, 牧区蒙古族人群54T等位基因频率亦明显增高, 且差异非常显著(χ2 = 34.23, P<0.01). 市区蒙古族和汉族相比较, 突变型等位基因频率无统计学差别(χ2 = 0.47, P>0.05).
饮食因素与冠心病、动脉粥样硬化、高脂血症等代谢性疾病的发生、发展关系密切, 业已证实, 西方的饮食方式, 即高脂饮食习惯是诱发此类疾病的重要因素之一. 20世纪末, 发现并初步证实IFABP基因在机体内脂类吸收、代谢中发挥着重要作用[2-14]. FABPs是一组细胞内小分子量蛋白超家族, Mr 15 000, 迄今为止, 共发现8种, 其分布具有组织特异性[15]. IFABP是这家族成员之一, 分布于小肠上皮细胞, 与食物中饱和、非饱和长链脂肪酸(long chain fatty acid, LCFA)的摄取、运输及代谢有关[16]. 1995年, Baier et al[17]报道, 人类IFABP基因第2外显子54位点存在点突变, 编码氨基酸由丙氨酸(Ala)突变为苏氨酸(Thr). 实验证实, 突变型T54 IFABP与配基LCFA的亲和力是野生型A54 IFABP的2倍[17]; 细胞学实验结果显示, 转染了54T/T IFABP基因的CaCo-2细胞摄取脂肪酸的能力, 分泌三酰甘油(TG)的浓度均高于对照组54A/A IFABP CaCo-2细胞[18]. 从结构上, 尽管54T与配基结合无明显的直接关系, 但是苏氨酸(T)替代丙氨酸(A), 使蛋白分子侧链增大, 影响了配基进出结构域"入口", 从而降低了IFABP运输配基的转移速率[19]. 一些学者认为, 突变型54T IFABP分子结构的微小改变, 可能影响了脂类的正常代谢. 最近临床研究结果显示, 与野生型54A相比较, 突变型54T携带者, 餐后血浆非酯化脂肪酸、TG、载脂蛋白E(ApoE)及胰岛素水平均显著升高, 并且持续时间明显延长[20-21]. 早在1992年, Patsch et al发现, 餐后高脂血与冠心病的发生、发展有显著的相关性[22]. 因此, 预测突变型54T IFABP可能与异常的脂类代谢相关疾病有关.
肠道IFABP基因表达受食物中LCFA正向调节[23], 从十二指肠到结肠, 从绒毛顶端到肠隐窝, 随着上皮细胞摄取脂肪酸能力的降低, IFABP基因表达呈明显递减的阶梯状分布. 随着食物中脂肪酸含量增加, 肠道上皮细胞IFABP表达亦增多. 是否高脂饮食也与IFABP基因点突变有关? 目前尚无确切答案. 但从另一个角度, Vincent et al[24]通过饮食干预实验发现, 高脂饮食引起的高三酰甘油血症与54T IFABP基因有直接关系; Hegele et al[25]研究证实, 高纤维饮食可降低54T基因携带者外周血LDL-胆固醇和ApoB浓度. 人类基因的多态性是普遍存在的. 业已证实, 许多代谢性疾病, 如心血管疾病、糖尿病、肥胖症等起因于遗传的易感性和环境因素的相互作用. 大量资料显示, 与冠心病相关的参与脂类代谢基因, 如载脂蛋白E, B, A-Ⅳ, C-Ⅲ, LDL受体和IFABP的多态性改变与饮食有关. 边远的牧区蒙古族人很大程度上仍保持着传统的游牧民族的生活习惯, 饮食主要以高脂的肉、奶类为主, 蔬菜类较少; 而市区蒙古族人的饮食习惯与汉族几乎没有区别, 营养成分更趋于多元化. 我们的实验结果显示, 在市区蒙古族人群和汉族人群中, 突变型54T IFABP基因分布频率无统计学差别(P>0.05), 并且两者的突变频率与其他种族报道类似[26]. 牧区蒙古族人群54T基因突变频率明显增高, 与前两者相比, 差异非常显著(P<0.01). 因此, 我们推测, 在市区蒙、汉族人群中, IFABP基因54A/T多态性频率分布可能与种族无关; 而高脂饮食习惯可能与牧区蒙古族人群突变型54T IFABP基因高频率分布有关.
脂类代谢异常与心血管疾病的发生密切相关, 环境与遗传因素的相互作用越来越引起关注. 目前的研究结果显示, IFABP基因第2外显子54Ala/Thr突变可能与腹性肥胖、高血脂、高血糖、胰岛素抵抗等代谢综合征有关. 本文探讨了不同种族、不同饮食习惯与等位基因54Ala/Thr频率分布的关系, 为今后进一步研究打下理论基础.
本文比较蒙汉人群IFABP Ala54Thr多态性频率, 观察生活习惯对他的影响, 有一定的创新性, 有较大的理论意义. 设计合理, 技术先进, 结果可靠.
电编: 张敏 编辑:潘伯荣
| 1. | Boord JB, Fazio S, Linton MF. Cytoplasmic fatty acid-binding proteins: emerging roles in metabolism and atherosclerosis. Curr Opin Lipidol. 2002;13:141-147. [PubMed] [DOI] |
| 2. | Ribalta J, Halkes CJ, Salazar J, Masana L, Cabezas MC. Additive effects of the PPARgamma, APOE, and FABP-2 genes in increasing daylong triglycerides of normolipidemic women to concen-trations comparable to those in men. Clin Chem. 2005;51:864-871. [PubMed] [DOI] |
| 3. | Tai ES, Corella D, Demissie S, Cupples LA, Coltell O, Schaefer EJ, Tucker KL, Ordovas JM. Polyunsa-turated fatty acids interact with the PPARA-L162V polymorphism to affect plasma triglyceride and apolipoprotein C-III concentrations in the Framingham Heart Study. J Nutr. 2005;135:397-403. [PubMed] |
| 4. | Georgopoulos A, Aras O, Tsai MY. Codon-54 poly-morphism of the fatty acid-binding protein 2 gene is associated with elevation of fasting and postpran-dial triglyceride in type 2 diabetes. J Clin Endocrinol Metab. 2000;85:3155-3160. [PubMed] |
| 5. | Georgopoulos A, Aras O, Noutsou M, Tsai MY. Unlike type 2 diabetes, type 1 does not interact with the codon 54 polymorphism of the fatty acid bind-ing protein 2 gene. J Clin Endocrinol Metab. 2002;87:3735-3739. [PubMed] [DOI] |
| 6. | Umpaichitra V, Banerji MA, Castells S. Postprandial hyperlipidemia after a fat loading test in minority adolescents with type 2 diabetes mellitus and obesi-ty. J Pediatr Endocrinol Metab. 2004;17:853-864. [PubMed] [DOI] |
| 7. | Galluzzi JR, Cupples LA, Meigs JB, Wilson PW, Schaefer EJ, Ordovas JM. Association of the Ala54-Thr polymorphism in the intestinal fatty acid-bind-ing protein with 2-h postchallenge insulin levels in the Framingham Offspring Study. Diabetes Care. 2001;24:1161-1166. [PubMed] [DOI] |
| 8. | Marin C, Perez-Jimenez F, Gomez P, Delgado J, Paniagua JA, Lozano A, Cortes B, Jimenez-Gomez Y, Gomez MJ, Lopez-Miranda J. The Ala54Thr poly-morphism of the fatty acid-binding protein 2 gene is associated with a change in insulin sensitivity after a change in the type of dietary fat. Am J Clin Nutr. 2005;82:196-200. [PubMed] |
| 9. | Lefevre M, Lovejoy JC, Smith SR, Delany JP, Champagne C, Most MM, Denkins Y, de Jonge L, Rood J, Bray GA. Comparison of the acute response to meals enriched with cis- or trans-fatty acids on glucose and lipids in overweight individuals with differing FABP2 genotypes. Metabolism. 2005;54:1652-1658. [PubMed] [DOI] |
| 10. | Salguero ML, Leon RE, Santos A, Roman S, Segura-Ortega JE, Panduro A. The role of FABP2 gene polymorphism in alcoholic cirrhosis. Hepatol Res. 2005;. [PubMed] [DOI] |
| 11. | Pollex RL, Hanley AJ, Zinman B, Harris SB, Hegele RA. Clinical and genetic associations with hypertriglyceridemic waist in a Canadian aboriginal population. Int J Obes (Lond). 2006;30:484-491. [PubMed] [DOI] |
| 12. | Gonzalez Sanchez JL, Serrano Rios M, Fernandez Perez C, Laakso M, Martinez Larrad MT. Effect of the Pro12Ala polymorphism of the peroxisome proliferator-activated receptor gamma-2 gene on adiposity, insulin sensitivity and lipid profile in the Spanish population. Eur J Endocrinol. 2002;147:495-501. [PubMed] [DOI] |
| 13. | Pollex RL, Hanley AJ, Zinman B, Harris SB, Khan HM, Hegele RA. Metabolic syndrome in aboriginal Canadians: prevalence and genetic associations. Atherosclerosis. 2006;184:121-129. [PubMed] [DOI] |
| 14. | Lindi V, Schwab U, Louheranta A, Laakso M, Vessby B, Hermansen K, Storlien L, Riccardi G, A Rivellese A. Impact of the Pro12Ala polymorphism of the PPAR-gamma2 gene on serum triacylglycerol response to n-3 fatty acid supplementation. Mol Genet Metab. 2003;79:52-60. [PubMed] [DOI] |
| 15. | Zimmerman AW, Veerkamp JH. Members of the fatty acid-binding protein family inhibit cell-free protein synthesis. FEBS Lett. 1998;437:183-186. [PubMed] [DOI] |
| 16. | Storch J, Thumser AE. The fatty acid transport function of fatty acid-binding proteins. Biochim Biophys Acta. 2000;1486:28-44. [PubMed] [DOI] |
| 17. | Baier LJ, Sacchettini JC, Knowler WC, Eads J, Paolisso G, Tataranni PA, Mochizuki H, Bennett PH, Bogardus C, Prochazka M. An amino acid substitution in the human intestinal fatty acid binding protein is associated with increased fatty acid binding, increased fat oxidation, and insulin resistance. J Clin Invest. 1995;95:1281-1287. [PubMed] [DOI] |
| 18. | Baier LJ, Bogardus C, Sacchettini JC. A polymor-phism in the human intestinal fatty acid binding protein alters fatty acid transport across Caco-2 cells. J Biol Chem. 1996;271:10892-10896. [PubMed] [DOI] |
| 19. | Ito K, Nakatani K, Fujii M, Katsuki A, Tsuchihashi K, Murata K, Goto H, Yano Y, Gabazza EC, Sumida Y. Codon 54 polymorphism of the fatty acid binding protein gene and insulin resistance in the Japanese population. Diabet Med. 1999;16:119-124. [PubMed] [DOI] |
| 20. | Zhang F, Lucke C, Baier LJ, Sacchettini JC, Hamilton JA. Solution structure of human intestinal fatty acid binding protein with a naturally-occurring single amino acid substitution (A54T) that is associated with altered lipid metabolism. Biochemistry. 2003;42:7339-7347. [PubMed] [DOI] |
| 21. | Nakanishi S, Yamane K, Kamei N, Okubo M, Kohno N. The effect of polymorphism in the intestinal fatty acid-binding protein 2 gene on fat metabolism is associated with gender and obesity amongst non-diabetic Japanese-Americans. Diabetes Obes Metab. 2004;6:45-49. [PubMed] [DOI] |
| 22. | Patsch JR, Miesenbock G, Hopferwieser T, Muhl-berger V, Knapp E, Dunn JK, Gotto AM Jr, Patsch W. Relation of triglyceride metabolism and coronary artery disease. Studies in the postprandial state. Arterioscler Thromb. 1992;12:1336-1345. [PubMed] [DOI] |
| 23. | Mochizuki K, Suruga K, Yagi E, Takase S, Goda T. The expression of PPAR-associated genes is modu-lated through postnatal development of PPAR subtypes in the small intestine. Biochim Biophys Acta. 2001;1531:68-76. [PubMed] [DOI] |
| 24. | Vincent S, Planells R, Defoort C, Bernard MC, Ger-ber M, Prudhomme J, Vague P, Lairon D. Genetic polymorphisms and lipoprotein responses to diets. Proc Nutr Soc. 2002;61:427-434. [PubMed] [DOI] |
| 25. | Hegele RA, Wolever TM, Story JA, Connelly PW, Jenkins DJ. Intestinal fatty acid-binding protein variation associated with variation in the response of plasma lipoproteins to dietary fibre. Eur J Clin Invest. 1997;27:857-862. [PubMed] [DOI] |
| 26. | Kim CH, Yun SK, Byun DW, Yoo MH, Lee KU, Suh KI. Codon 54 polymorphism of the fatty acid binding protein 2 gene is associated with increased fat oxidation and hyperinsulinemia, but not with intestinal fatty acid absorption in Korean men. Metabolism. 2001;50:473-476. [PubMed] [DOI] |