修回日期: 2006-03-10
接受日期: 2006-03-20
在线出版日期: 2006-05-08
目的: 探讨Stat5反义寡核苷酸(Stat5 AS-ON)联合5-氟尿嘧啶(5-FU)治疗胃癌的作用机制.
方法: Stat5 AS-ON、5-FU单用或联用处理胃癌细胞BGC823, Western blot检测Stat5、p-Stat5、cyclin D1与Bcl-xL表达, MTT法检测细胞增殖状态, 流式细胞技术检测细胞周期与凋亡.
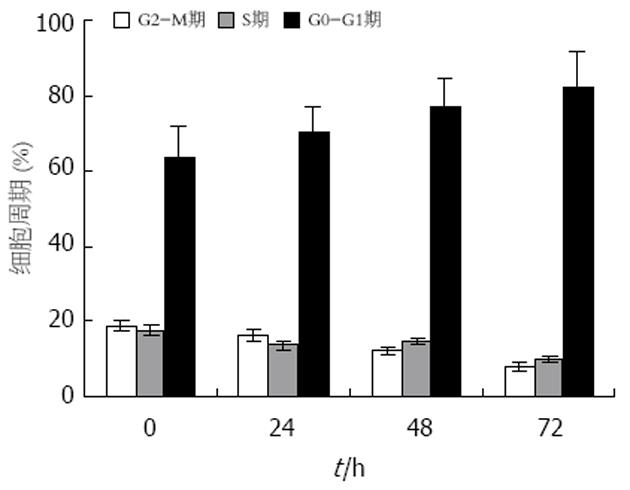
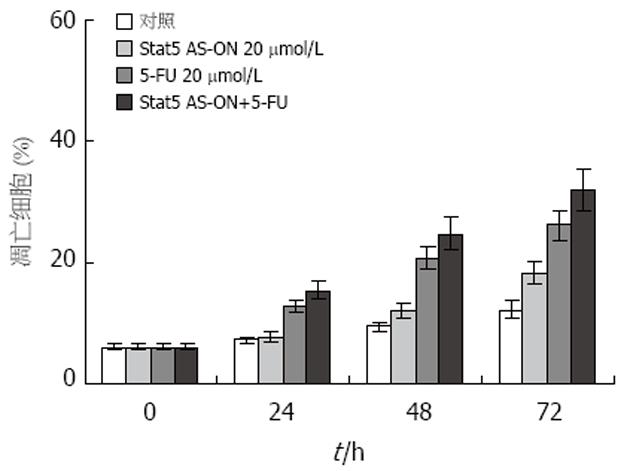
结果: Stat5 AS-ON与5-FU作用于BGC823细胞72 h后, G1期细胞比率由65.7%上升至78.2%, S期细胞比率分别由18.6%, 下降至10.5%, 凋亡细胞百分比由7.4%增加至21.6%. Stat5 AS-ON与5-FU可以抑制胃癌细胞增殖, 促进胃癌细胞凋亡, 联合应用Stat5 AS-ON与5-FU可以起协同作用, 明显抑制胃癌细胞Stat5信号转导通路活化.
结论: 选择性阻断细胞内信号转导通路可能为治疗胃癌提供新途径.
引文著录: 马向涛, 余力伟, 王杉, 杜如昱, 崔志荣. Stat5反义寡核苷酸联合5-氟尿嘧啶对胃癌细胞增殖与凋亡的影响. 世界华人消化杂志 2006; 14(13): 1257-1261
Revised: March 10, 2006
Accepted: March 20, 2006
Published online: May 8, 2006
AIM: To investigate the mechanism of Stat5 antisense oligonucleotide (Stat5 AS-ON) combined with 5-fluorouracil (5-FU) in the treatment of gastric cancer.
METHODS: Human gastric cancer cell line BGC823 was treated with Stat5 AS-ON and 5-FU, respectively, or in combination. The expression of Stat5, p-Stat5, cyclin D1 and Bcl-xL in the cells were detected by Western blot, and the cell cycle and apoptosis were detected by flow cytometry.
RESULTS: After treatment with Stat5 AS-ON and 5-FU for 72 h, the ratio of G1-phase cells was up-regulated from 65.7% to 78.2%, and that of S-phase cells was down-regulated from 18.6% to 10.5%; the percentage of apoptotic cells was increased from 7.4% to 21.6%. Stat5 AS-ON and 5-FU synergically inhibited the growth of gastric cancer cells, induced significant apoptosis of the cancer cells, and they reduced the expression and phosphorylation of Stat5, as well as the expression of cyclin D1 and Bcl-xL.
CONCLUSION: Selective inhibition of specific signaling pathway in the cells may provide a new approach in the treatment of gastric cancer.
- Citation: Ma XT, Yu LW, Wang S, Du RY, Cui ZR. Effects of Stat5 antisense oligonucleotide combined with 5-fluorouracil on proliferation and apoptosis of gastric cancer cells. Shijie Huaren Xiaohua Zazhi 2006; 14(13): 1257-1261
- URL: https://www.wjgnet.com/1009-3079/full/v14/i13/1257.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v14.i13.1257
Stat5是转录信号传导子与激活子通路(signal transducers and activators of transcription, STATs)的重要成员, 该通路接受生长因子与细胞因子等细胞外信号刺激, 调节细胞增殖、分化及凋亡[1]. 目前已发现Stat5在白血病、头颈部鳞癌及乳腺癌等多种肿瘤组织及细胞系中呈持续活化[2-4], 但是关于Stat5在调控胃癌细胞凋亡过程中的作用还有待进一步研究. 我们应用Stat5反义寡核苷酸与5-氟尿嘧啶处理胃癌细胞, 观察对胃癌细胞增殖和凋亡的影响, 探讨以Stat5信号转导通路为靶点在药物治疗胃癌中的作用.
人胃癌细胞BGC823, 培养于含有胎牛血清(美国HyClone公司)的RPMI 1640培养基(美国Gibcol公司). Stat5反义寡核苷酸根据Stat5翻译起始点合成, 同时本研究还设立了正义寡核苷酸链和错配寡核苷酸链作为对照组, 错配链的序列在GenBank数据库进行同源性检索, 未发现同源序列. (1)Stat5反义寡核苷酸序列: 5'CCA CAC AGC CAT GTT TAC CCG 3'; (2)Stat5正义寡核苷酸序列: 5'CGG GTA AAC ATG GCT GTG TGG 3'; (3)Stat5错配寡核苷酸序列: 5'CCA CAG AGC CAT GTT TTC CCG 3'. 以上寡核苷酸由美国Santa Cruz公司合成及纯化. 阳离子脂质体LipofectAmine2000(美国Gibcol公司), 转染过程参照Gibcol公司手册.
分为对照组、Stat5 AS-ON组、5-FU组、5-FU+Stat5 AS-ON组. 溶液中含等量乙醇和DMSO, 各组溶液的乙醇或DMSO不超过1 mL/ L. 接种细胞于96孔板, 贴壁后, 无血清培养细胞16-24 h, 使细胞同步化. 空白对照组加无血清培养基, 试验组分别加入Stat5 AS-ON和/或5-FU, 0, 24, 48, 72 h分别加入5 g/L MTT(美国Sigma公司), 继续培养4 h, 每孔加入DMSO 200 μL, 酶标仪测定540 nm吸光度A, 绘制生长曲线. 无血清培养细胞16-24 h, 使同步化, 空白对照组加无血清培养基, 试验组分别加入Stat5 AS-ON和/或5-FU继续培养, 0, 24, 48, 72 h分别消化细胞, PBS 0.5 mol/L重悬细胞, 700 mL/L冰乙醇固定细胞过夜, 加入RNAase A至终浓度50 mg/L, 37℃恒温水浴1 h, 加入PI(美国Sigma公司)至终浓度50 mg/L, 4℃避光染色1 h, 上流式细胞仪FACScan(美国Becton-Dickinson公司)检测, 资料用Cell Quest细胞周期分析软件处理. 细胞凋亡同步化处理及单细胞悬液制备同前, 应用凋亡检测试剂盒, PBS 0.5 mol/L离心洗涤2次, 200 μL结合缓冲液重悬, 加入ANNEXTIN V-FITC至终浓度1 mg/L, 加入PI至终浓度2.5 mg/L, 室温避光染色15 min, 上流式细胞仪FACScan(美国Becton-Dickinson公司)检测. (1)细胞总蛋白提取: 细胞于裂解缓冲液中裂解(150 mmol/L氯化钠; 10 g/L过氧胆酸钠; 10 g/L Triniton X-100; 1 g/L十二烷基磺酸钠; 10 mmol/L Tris, pH 7.2; 1 mmol/L正钒酸钠; 1 mmol/L苯甲磺酰氟; 1 mmol/L氟化钠; 0.1 mmol/L抑肽酶, 1 mmol/L亮抑蛋白酶肽). 裂解液在4℃条件下13 000 r/min离心30 min, 收集上清液得到细胞总蛋白; (2)胞质蛋白提取: 收集细胞悬液, 4℃条件下13 000 r/min离心2 min; 胞质缓冲液(20 mmol/L羟乙基哌嗪乙磺酸, pH 7.9; 10 mmol/L氯化钾; 100 mL/L甘油; 1 mmol/L乙二胺四乙酸; 1 mmol/L二硫苏糖醇; 0.1 mmol/L正钒酸钠; 1 mmol/L苯甲磺酰氟)重悬, 裂解产物4℃条件下13 000 r/min离心5 min, 收集上清液得到胞质蛋白. (3)细胞核蛋白提取: 收集细胞悬液, 用低渗缓冲液于冰上裂解细胞10 min; 4℃条件下13 000 r/min离心1 min; 4℃条件下用高盐缓冲液(420 mmol/L氯化钠; 20 mmol/L羟乙基哌嗪乙磺酸, pH 7.9; 10 mmol/L氯化钾; 200 mL/L甘油; 1 mmol/L乙二胺四乙酸; 1 mmol/L二硫苏糖醇; 0.1 mmol/L正钒酸钠; 1 mmol/L苯甲磺酰氟)重悬粗提的细胞核, 振荡30 min; 4℃条件下13 000 r/min离心10 min; 取上清为核提取物, 贮存于-80℃冰箱. 以牛血清蛋白(BSA)作为标准品, 根据蛋白定量试剂盒(美国Bio-Rad公司)手册绘制蛋白定量标准曲线, 于分光光度计595 nm下测吸光度A值, 计算提取液蛋白浓度. 在进行Western blot之前将蛋白提取物与十二烷基磺酸钠(SDS)上样缓冲液按1∶1混合(125 mmol/L Tris HCl, pH 6.8; 40 g/L十二烷基磺酸钠; 200 mL/L甘油; 100 g/L 2-巯基乙醇)后100℃水浴下加热5 min. 取蛋白样品50 μg, 75-100 g/L聚丙烯酰胺凝胶电泳分离后电转移到PVDF膜上. 电泳时在聚丙烯酰胺凝胶中加入预染标准分子量蛋白作为指示. 转膜后用TBST缓冲液(10 mmol/L Tris HCl, pH 7.5, 150 mmol/L 氯化钠, 5 g/L Tween-20)与5 g/L牛血清白蛋白封闭30 min. 封闭后, 加入一抗(Stat5, p-Stat5, Bcl-2, Bcl-xL, Mcl-1, Caspase-3, GAPDH)(美国Santa Cruz公司), 工作浓度1∶1000; GAPDH作为内参照, 于4℃条件下孵育过夜, 用TBST(每次5 min)洗膜后, 与辣根过氧化物酶结合的二抗(英国Amersham公司)孵育30 min, 工作浓度1∶1000. 然后用ECL化学发光试剂盒检测杂交信号. 细胞核蛋白样品50 μg上样于75 g/L的聚丙烯酰胺凝胶经电泳分离后, 电转移至PVDF膜(美国Millipore公司), 封闭后, 辣根过氧化物酶结合的二抗, (英国Amersham公司), 工作浓度1∶1000. 用ECL(英国Amersham公司)化学发光试剂盒检测杂交信号. 用PhosphoImager图像分析仪(美国Molecular Dynamics公司)测定条带的吸光度A值, 以A值代表蛋白的相对表达量.
统计学处理 应用SPSS 12.0统计学软件, 采用独立样本t检验, P<0.05时为有统计学差异.
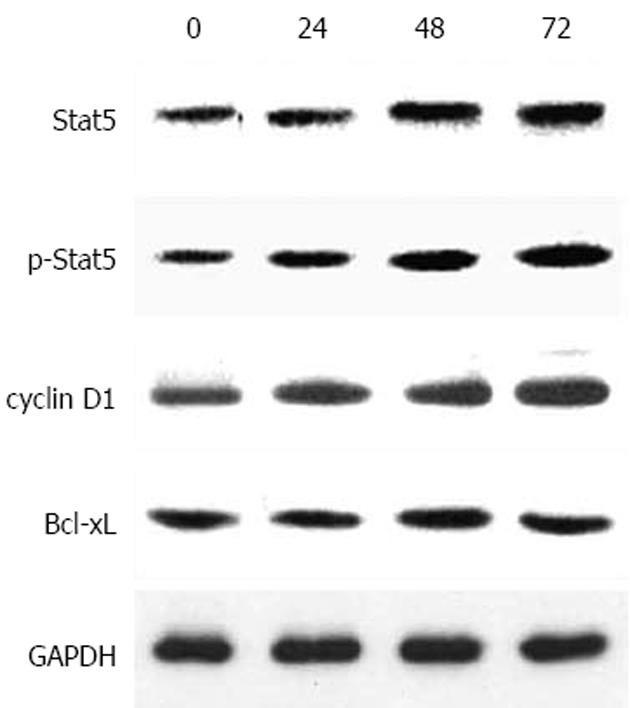
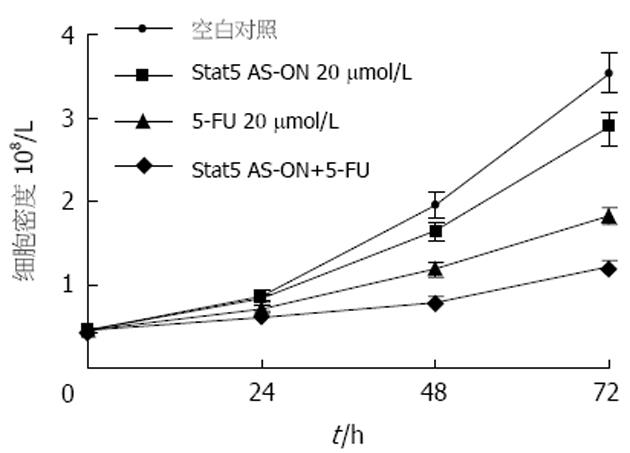
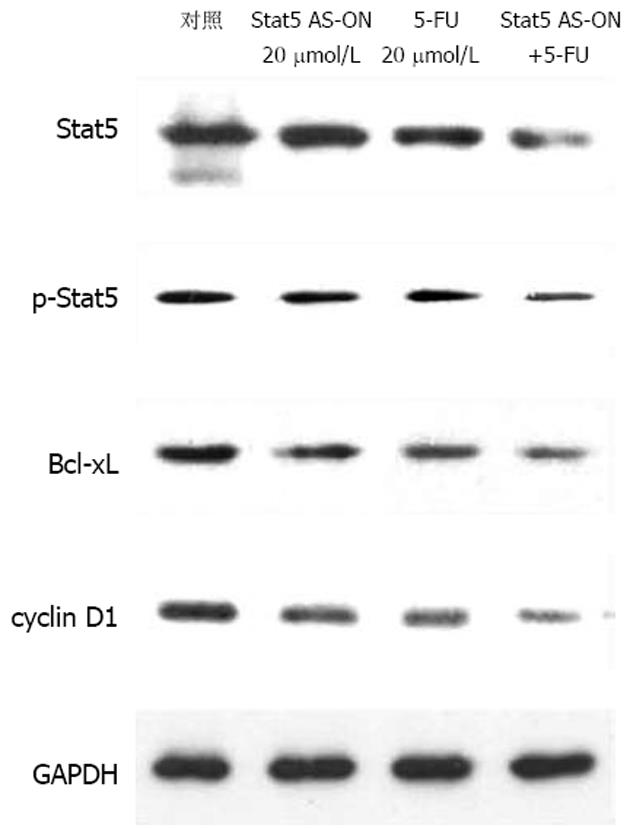
Stat5在胃癌细胞中持续活化(图1). Stat5 AS-ON与5-FU作用于BGC823细胞后, 细胞增殖水平下降(图2). Stat5 AS-ON与5-FU作用于BGC823细胞72 h后, G1期细胞比率由65.7%上升至78.2%, S期细胞比率由18.6%下降至10.5%, 细胞增殖受抑制(图3). Stat5 AS-ON与5-FU作用于BGC823细胞72 h后, 凋亡细胞百分比由7.4%增加至21.6%(图4). Stat5 AS-ON与5-FU作用于BGC823细胞72 h后, Stat5表达与活性下调, 其靶基因产物Bcl-xL与cyclin D1表达下降(图5).
STATs信号转导通路与细胞的增殖、分化及凋亡关系密切, 该通路异常活化可导致细胞异常增殖和恶性转化. 哺乳动物中STATs家族由7个成员组成: Stat1-Stat4, Stat5a, Stat5b及Stat6[2]. Stat5最初被称为泌乳素诱导的乳腺因子(mammary gland factor, MGF), 在乳腺上皮细胞增殖与分化中起重要作用[5]. Stat5包括Stat5a与Stat5b两种异构体, 结构上具有95%的同源性. 研究证实Stat5表达与活化不仅与乳腺癌发生、发展密切相关, 而且在其他肿瘤如髓样白血病、头颈部鳞状细胞癌及前列腺癌中异常表达与活化[5-10]. 其中Stat5作为上游酪氨酸激酶通过调控靶基因而诱导某些关键产物的表达来影响肿瘤的发生, 重要的靶基因产物包括影响细胞凋亡的Bcl-2家族成员[11-14]. Bcl-2家族包括抑凋亡和促凋亡两大类, 前者包括Bcl-2, Bcl-xL, Mcl-1等, 后者包括Bax, Bak, Bcl-xS等. bcl-x基因启动子上存在多个STATs结合位点, STATs可直接与bcl-x启动子结合而启动转录[14-17]. Gutierrez-Castellanos[18]在研究慢性髓性白血病(chronic myelogenous leukemia, CML)时发现, CML患者外周血单核细胞中Bcl-xL表达在慢性期下降, 而在急变期迅速升高, 逆转录聚合酶链反应(RT-PCR)显示在此过程中Bcl-xL转录受到磷酸化Stat5的调控.
本研究结果显示, Stat5通路在胃癌细胞BGC823增殖过程中持续激活, 应用Stat5 AS-ON作用于胃癌细胞系BGC823, 发现BGC823细胞增殖水平随Stat5 AS-ON作用时间延长而下降, 相应空白对照组变化不明显. 由于胃癌对化疗药物属中低度敏感, 即使最有效的5-FU其反应率仅有20%-30%, 对其耐药机制的研究仍是热点和难点, 已经有研究显示STATs信号转导通路异常激活与肿瘤耐药可能有关, 但是具体机制尚不清楚[19-23]. 为研究Stat5在介导胃癌药物治疗的作用机制, 我们应用Stat5 AS-ON, Stat5 AS-ON+5-FU分别作用于BGC823细胞, 发现BGC823细胞增殖水平随药物作用时间延长而下降, 以Stat5 AS-ON+5-FU组细胞下降为明显, Stat5 AS-ON+5-FU作用于BGC823细胞72 h后, 细胞增殖受抑制, 凋亡细胞增加. Western blot显示, BGC823细胞中Stat5, p-Stat5, Bcl-xL及cyclin D1表达水平明显下降, 提示胃癌细胞耐药可能与Stat5异常激活有关, Stat5 AS-ON可以协同化疗药物5-FU对胃癌细胞BGC823起治疗作用.
总之, Stat5信号转导通路在胃癌细胞中的转录调控机制尚不清楚, Stat5的异常激活与胃癌细胞凋亡关系还有待于进一步明确[24-27]. 实验动物模型及临床观察中发现: 肿瘤细胞耐受化疗与Stat5与Bcl-2成员异常增高有关, 阻断Stat5通路可诱导耐药肿瘤细胞凋亡[28-30]. 深入研究Stat5信号转导通路作用机制有可能为治疗胃癌提供新的理论和实验基础[31-33].
Stat5在白血病、头颈部鳞癌及乳腺癌等多种肿瘤组织及细胞系中呈持续活化, 但是关于Stat5在调控胃癌细胞凋亡过程中的作用还有待进一步研究.
Stat5作为上游激酶通过调控靶基因转录而影响肿瘤细胞的增殖与凋亡机制是近期研究的重点.
关于Stat5信号转导通路的研究主要集中于血液系统与乳腺肿瘤, 而本文研究的Stat5在胃癌细胞耐药中的作用机制是目前研究发展的趋势.
本文为研究肿瘤耐药机制提供了新的研究靶点, 进一步研究可通过阻断Stat5通路了解肿瘤细胞侵袭等转移相关特征的改变.
本文内容较新, 结果可信, 对于胃癌细胞耐药机制从信号转导通路角度进行了探讨, 为进一步研究提供了理论与实验基础.
电编: 李琪 编辑:潘伯荣
| 1. | Haura EB, Turkson J, Jove R. Mechanisms of disease: Insights into the emerging role of signal transducers and activators of transcription in cancer. Nat Clin Pract Oncol. 2005;2:315-324. [PubMed] [DOI] |
| 2. | Redell MS, Tweardy DJ. Targeting transcription factors for cancer therapy. Curr Pharm Des. 2005;11:2873-2887. [PubMed] [DOI] |
| 3. | Scherr M, Chaturvedi A, Battmer K, Dallmann I, Schultheis B, Ganser A, Eder M. Enhanced sensitivity to inhibition of SHP2, STAT5, and Gab2 expression in chronic myeloid leukemia (CML). Blood. 2006;107:3279-3287. [PubMed] [DOI] |
| 4. | Debierre-Grockiego F. Anti-apoptotic role of STAT5 in haematopoietic cells and in the pathogenesis of malignancies. Apoptosis. 2004;9:717-728. [PubMed] [DOI] |
| 5. | Lai SY, Childs EE, Xi S, Coppelli FM, Gooding WE, Wells A, Ferris RL, Grandis JR. Erythropoietin-mediated activation of JAK-STAT signaling contributes to cellular invasion in head and neck squamous cell carcinoma. Oncogene. 2005;24:4442-4449. [PubMed] [DOI] |
| 6. | Nikitakis NG, Siavash H, Sauk JJ. Targeting the STAT pathway in head and neck cancer: recent advances and future prospects. Curr Cancer Drug Targets. 2004;4:637-651. [PubMed] [DOI] |
| 7. | Clevenger CV. Roles and regulation of stat family transcription factors in human breast cancer. Am J Pathol. 2004;165:1449-1460. [PubMed] [DOI] |
| 8. | Shan L, Yu M, Clark BD, Snyderwine EG. Possible role of Stat5a in rat mammary gland carcinogenesis. Breast Cancer Res Treat. 2004;88:263-272. [PubMed] [DOI] |
| 9. | Sultan AS, Xie J, LeBaron MJ, Ealley EL, Nevalainen MT, Rui H. Stat5 promotes homotypic adhesion and inhibits invasive characteristics of human breast cancer cells. Oncogene. 2005;24:746-760. [PubMed] [DOI] |
| 10. | Buitenhuis M, Coffer PJ, Koenderman L. Signal transducer and activator of transcription 5 (STAT5). Int J Biochem Cell Biol. 2004;36:2120-2124. [PubMed] [DOI] |
| 11. | Li H, Zhang Y, Glass A, Zellweger T, Gehan E, Bubendorf L, Gelmann EP, Nevalainen MT. Activation of signal transducer and activator of transcription-5 in prostate cancer predicts early recurrence. Clin Cancer Res. 2005;11:5863-5868. [PubMed] [DOI] |
| 12. | Feldman L, Wang Y, Rhim JS, Bhattacharya N, Loda M, Sytkowski AJ. Erythropoietin stimulates growth and STAT5 phosphorylation in human prostate epithelial and prostate cancer cells. Prostate. 2006;66:135-145. [PubMed] [DOI] |
| 13. | Li H, Ahonen TJ, Alanen K, Xie J, LeBaron MJ, Pretlow TG, Ealley EL, Zhang Y, Nurmi M, Singh B. Activation of signal transducer and activator of transcription 5 in human prostate cancer is associated with high histological grade. Cancer Res. 2004;64:4774-4782. [PubMed] [DOI] |
| 14. | Cory S, Huang DC, Adams JM. The Bcl-2 family: roles in cell survival and oncogenesis. Oncogene. 2003;22:8590-8607. [PubMed] [DOI] |
| 15. | Coultas L, Strasser A. The role of the Bcl-2 protein family in cancer. Semin Cancer Biol. 2003;13:115-123. [PubMed] [DOI] |
| 16. | Moucadel V, Constantinescu SN. Differential STAT5 signaling by ligand-dependent and constitutively active cytokine receptors. J Biol Chem. 2005;280:13364-13373. [PubMed] [DOI] |
| 17. | Menon MP, Karur V, Bogacheva O, Bogachev O, Cuetara B, Wojchowski DM. Signals for stress erythropoiesis are integrated via an erythropoietin receptor-phosphotyrosine-343-Stat5 axis. J Clin Invest. 2006;116:683-694. [PubMed] [DOI] |
| 18. | Gutierrez-Castellanos S, Cruz M, Rabelo L, Godinez R, Reyes-Maldonado E, Riebeling-Navarro C. Differences in BCL-X(L) expression and STAT5 phosphorylation in chronic myeloid leukaemia patients. Eur J Haematol. 2004;72:231-238. [PubMed] [DOI] |
| 19. | Gonzalez RJ, Mansfield PF. Adjuvant and neoadjuvant therapy for gastric cancer. Surg Clin North Am. 2005;85:1033-1051. [PubMed] [DOI] |
| 20. | Lim L, Michael M, Mann GB, Leong T. Adjuvant therapy in gastric cancer. J Clin Oncol. 2005;23:6220-6232. [PubMed] [DOI] |
| 21. | Klampfer L. Signal transducers and activators of transcription (STATs): Novel targets of chemopreventive and chemotherapeutic drugs. Curr Cancer Drug Targets. 2006;6:107-121. [PubMed] [DOI] |
| 22. | Turkson J. STAT proteins as novel targets for cancer drug discovery. Expert Opin Ther Targets. 2004;8:409-422. [PubMed] [DOI] |
| 23. | Adachi S, Leoni LM, Carson DA, Nakahata T. Apoptosis induced by molecular targeting therapy in hematological malignancies. Acta Haematol. 2004;111:107-123. [PubMed] [DOI] |
| 24. | To KF, Chan MW, Leung WK, Ng EK, Yu J, Bai AH, Lo AW, Chu SH, Tong JH, Lo KW. Constitutional activation of IL-6-mediated JAK/STAT pathway through hypermethylation of SOCS-1 in human gastric cancer cell line. Br J Cancer. 2004;91:1335-1341. [PubMed] [DOI] |
| 25. | Pai R, Lin C, Tran T, Tarnawski A. Leptin activates STAT and ERK2 pathways and induces gastric cancer cell proliferation. Biochem Biophys Res Commun. 2005;331:984-992. [PubMed] [DOI] |
| 26. | Yu LF, Cheng Y, Qiao MM, Zhang YP, Wu YL. Activation of STAT3 signaling in human stomach adenocarcinoma drug-resistant cell line and its relationship with expression of vascular endothelial growth factor. World J Gastroenterol. 2005;11:875-879. [PubMed] [DOI] |
| 27. | Song H, Sondak VK, Barber DL, Reid TJ, Lin J. Modulation of Janus kinase 2 by cisplatin in cancer cells. Int J Oncol. 2004;24:1017-1026. [PubMed] [DOI] |
| 28. | Alas S, Bonavida B. Inhibition of constitutive STAT3 activity sensitizes resistant non-Hodgkin's lymphoma and multiple myeloma to chemothe-rapeutic drug-mediated apoptosis. Clin Cancer Res. 2003;9:316-326. [PubMed] |
| 29. | Ma XT, Wang S, Ye YJ, Du RY, Cui ZR, Somsouk M. Constitutive activation of Stat3 signaling pathway in human colorectal carcinoma. World J Gastroenterol. 2004;10:1569-1573. [PubMed] [DOI] |
| 30. | Real PJ, Sierra A, De Juan A, Segovia JC, Lopez-Vega JM, Fernandez-Luna JL. Resistance to chemotherapy via Stat3-dependent overexpression of Bcl-2 in metastatic breast cancer cells. Oncogene. 2002;21:7611-7618. [PubMed] [DOI] |
| 31. | Wittig I, Groner B. Signal transducer and activator of transcription 5 (STAT5), a crucial regulator of immune and cancer cells. Curr Drug Targets Immune Endocr Metabol Disord. 2005;5:449-463. [PubMed] [DOI] |
| 32. | Buettner R, Mora LB, Jove R. Activated STAT signaling in human tumors provides novel molecular targets for therapeutic intervention. Clin Cancer Res. 2002;8:945-954. [PubMed] |
| 33. | Ma XT, Yu LW, Wang S, Zhang H, Du RY, Cui ZR. Molecular mechanism of cyclooxygenase-2 inhibitor in inhibition of proliferation of colon cancer cells by modulating Stat5 signal transduction pathway. Zhonghua Yixue Zazhi. 2005;85:2566-2569. [PubMed] |