修回日期: 2005-02-01
接受日期: 2005-02-26
在线出版日期: 2005-05-01
目的: 探讨CD44v6和MMP-2在食管癌组织中的表达及其与食管癌侵袭转移和预后的关系.
方法: 回顾性分析43例术前未进行化疗和放疗的食管癌的切除标本, 采用免疫组化S-P法检测食管癌组织中的CD44v6和MMP-2的表达.
结果: 食管癌中CD44v6的阳性表达率62.7%(27/43), MMP-2的阳性表达率为65.1%(28/43), CD44v6及 MMP-2的阳性表达与淋巴结的转移, 食管癌的病理分 以及手术后血性转移显著相关(P<0.05), 而且, CD44v6阳性表达者3 a生存率为25.62%, 5 a生存率为6.41%, 阴性表达3 a生存率为64.71%, 5 a生存率为56.82%, 二者之间差异显著(P = 0.000); MMP-2阳性者3 a生存率为18.8%, 5 a生存率为8.67%, 阴性表达者3 a生存率为66.30%, 5 a生存率为42.66%, 二者之间差异显著(P = 0.0 067); CD44v6的阳性表达和MMP-2的阳性表达显著性相关(P = 0.007).
结论: CD44v6和MMP-2对于食管鳞癌的侵袭、淋巴结转移、手术后血性转移以及预后有一定作用.
引文著录: 张林, 孟龙, 王磊, 彭忠民, 杜贾军, 王晓航, 刘莹, 陈景寒. 食管鳞癌MMP-2和CD44v6表达与侵袭、转移及预后之间的关系. 世界华人消化杂志 2005; 13(9): 1074-1077
Revised: February 1, 2005
Accepted: February 26, 2005
Published online: May 1, 2005
AIM: To detect the expression of CD44v6 and MMP-2 in esophagus squamous cell carcinoma, and to explore their association with invasion, metastasis and prognosis.
METHODS: A rapid immunohistochemical method (streptoavidin-peroxidase, SP) was used to detect CD44v6 and MMP-2 protein in 43 cases of esophagus squamous cell carcinoma treated without radiotherapy or chemotherapy before operation.
RESULTS: The expression rates of CD44v6 and MMP-2 in esophagus squamous cell carcinoma were 62.7% (27/43) and 65.1% (28/43), respectively. The positive expression of MMP-2 and CD44v6 were significantly correlated with lymph node metastasis, TNM stage and postoperative hematogenous metastasis (P<0.05). The 3-and 5-year survival rates of CD44v6 positive patients were 25.62% and 6.41%. The 3-and 5-year survival rates of CD44v6 negative patients were 64.71% and 56.82%. There was significant difference for survival time between CD44v6 positive and CD44v6 negative patients (P = 0.000). The 3- and 5-year survival rates of MMP-2 positive patients were 18.8% and 8.60%. The 3-and 5-year survival rates of MMP-2 negative patients were 66.30% and 42.82%. There was significant difference between MMP-2 positive and MMP-2 negative groups (P = 0.0067). CD44v6 positive expression was significantly correlated with MMP-2 positive expression (P = 0.007).
CONCLUSION: CD44v6 and MMP-2 may play a role in esophagus squamous cell carcinoma invasion, lympha node metastasis, and postoperative hematogenous metastasis as well as the prognosis of the patients.
- Citation: Zhang L, Meng L, Wang L, Peng ZM, Du JJ, Wang XH, Liu Y, Chen JH. Expression of CD44v6 and MMP-2 in esophagus squamous cell carcinoma and their association with invasion, metastasis and prognosis. Shijie Huaren Xiaohua Zazhi 2005; 13(9): 1074-1077
- URL: https://www.wjgnet.com/1009-3079/full/v13/i9/1074.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v13.i9.1074
基质金属蛋白酶(matrix metalloproteinase, MMPs)能够降解细胞外基质和基底膜成分, 破坏基底膜的完整性为肿瘤的浸润和转移提供了先决条件[1-14]. 细胞黏附因子(cell adhesion moleculars, CAMs)参与细胞的信号传导与活化, 与肿瘤的生长分化尤其是与肿瘤的浸润转移密切相关. CD44v6是细胞黏附因子的一种, 目前研究表明CD44与肿瘤的关系密切[15-24]. 我们利用免疫组织化学方法检测食管鳞癌组织MMP-2 和CD44v6的表达情况, 探讨这两种基因之间及其与食管鳞癌生物学行为, 特别是与食管癌浸润、转移、复发和预后之间的关系.
我科1996/1999年手术切除的食管鳞癌标本43例, 所有病例术前未做任何形式的化疗和放疗, 男25例, 女18例, 年龄35-72岁(平均55岁), 按照国际标准进行分期 Ⅰ(ⅠA+ⅠB)期17例, Ⅱ(ⅡA+ⅡB)期12例, Ⅲ(ⅢA+ⅢB)期14例. 淋巴结转移情况采用目前的国际分期, 在分期中, N0代表淋巴结无转移, N1、N2代表淋巴结转移, 只是转移的部位不同. 所有患者接受了食管癌切除胃食管吻合术和淋巴结清扫, 术后均严格随访, 无失访. 死亡31例, 存活12例, 5 a生存率为27.91%. 死亡的患者中有21例发生血性转移, 其中脑转移3例, 肝转移8例, 骨骼和脊柱转移6例, 肾上腺转移1例, 肺转移3例. 所有标本切取肿瘤标本蜡块.鼠抗人MMP-2mAb, 鼠抗人CD44v6mAb, 即用型S-P试剂盒, DAB二氨基联苯胺显色剂试剂盒, 以上试剂均购自福州迈新公司产品.
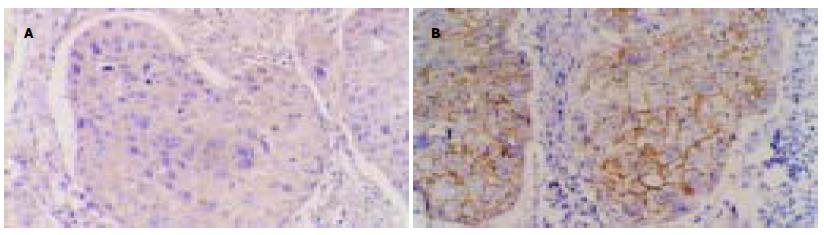
采用S-P法. MMP-2结果判定: MMP-2阳性结果判定, 低倍镜下见细胞质内出现明确的棕黄色颗粒的细胞为阳性. 合并计算5个不同视野染色细胞百分数及染色强度, 作评分, 0和1为阴性表达, 2和3为阳性表达. CD44v6结果判定CD44v6阳性产物分布于细胞膜上, 为粗细一致的棕黄色颗粒. 根据显色强度和阳性细胞数进行分级, 阴性(-)为整张组织切片均无着色; 弱阳性(+)为浅棕色, 阳性细胞计数<10%; 阳性(++)为棕色, 阳性细胞计数在10%-50%; 强阳性(+++)为深棕色, 阳性细胞计数>50%.
统计学处理 采用χ2检验, 精确概率法和Kaplan-meier 方法及Log Rank 检验进行生存分析. Spearman 等级相关分析,
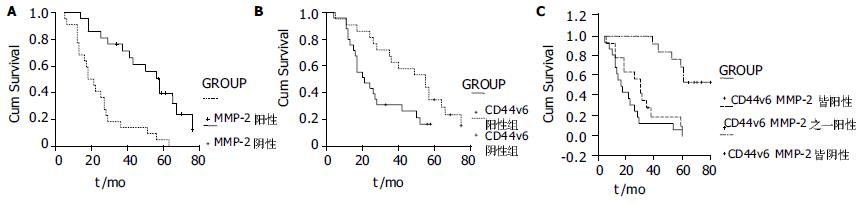
鳞状细胞癌中阳性表达为65.1%, 无淋巴结转移的患者中, MMP-2的阳性率为33.3%, 而N1+N2组的MMP-2的阳性率为92.0%, 两组之间差异显著(χ2 = 16.404, P = 0.000, 表1). 说明MMP-2和淋巴结转移密切相关.Ⅰ期17例, 其中MMP-2阳性率为41.2%, Ⅱ期+Ⅲ期26例, 阳性率为84.6%, 两组之间差异显著(χ2 = 8.833, P = 0.003, 表1). 说明食管癌临床分期越晚, MMP-2的阳性表达越高.转移组中, MMP-2阳性表达率为85.71%, 显著高于无转移复发组的(50.0%)差异显著(χ2 = 6.241, P = 0.012, 表1).MMP-2阳性表达者5 a生存率为6.4%, MMP-2阴性者5 a生存率为56.8%, 两组之间生存率之间经过log-rank检验, 具有显著性差异(P = 0.0 067, 图2A).
淋巴结无转移者CD44v6阳性率为33.3%, 而N1+N2组的CD44v6的阳性率为84.0%, 两组之间差异显著(χ2 = 11.499, P = 0.001, 表1).Ⅰ期17例, CD44v6阳性率为35.3%, Ⅱ期+Ⅲ食管癌26例, 阳性率为80.8%, 两组之间差异显著(χ2 = 13.858, P = 0.000, 表1). 转移组CD44v6阳性表达率为81.0%显著高于无转移复发组CD44v6的阳性率(χ2 = 5.795, P = 0.016, 表1)CD44v6阳性表达者5 a生存率为10.7%, CD44v6阴性者5 a生存率为53.5%, 二者差异显著(P = 0.0 000, 图2B).
MMP-2和CD44v6同时阳性的例数为22例, MMP-2表达阳性组CD44v6阳性例数明显高于MMP-2表达阴性组CD44v6阳性例数(P<0.05), 经过speraman等级相关分析发现食管癌中MMP-2表达与CD444v6表达成正相关(r = 0.785, P<0.01, 表2), 二者同时阳性患者生存期较差, 3 a生存期为10.53%, 5 a生存率为0%(图2C)
MMP-2能够分解基底膜的主要成分-Ⅳ型胶原蛋白, 而Ⅳ型胶原蛋白又是ECM的主要成分, 故称为Ⅳ型胶原酶, MMP-2(明胶酶A/72 ku Ⅳ胶原酶)能够分解基底膜的主要成分纤维连接蛋白和层连蛋白.本研究表明, MMP-2在食管癌组织中高表达这与其他研究报告相一致, 本组食管鳞癌阳性率为65.1%, MMP-2与食管鳞癌的临床病例特征密切相关, 淋巴结无转移的患者MMP-2阳性率为33.3%, 而淋巴结转移组的MMP-2的阳性率为92.0% 二者之间有显著性差异. 说明MMP-2和淋巴结转移密切相关, 而且随着分期的升高, 食管鳞癌组中MMP-2的阳性表达率明显增高, 在Ⅰ期中阳性率为41.2%, Ⅱ期和Ⅲ期中的阳性率为 84.6%, 两组之间差异显著(P<0.05). 术后发生转移的21例中有18例为MMP-2阳性, 无转移的22例患者中11例阳性, 差异显著. MMP-2能够促进肿瘤浸润转移所以影响了患者的预后, 我们发现MMP-2阴性组的3、5 a生存率为66.3%和42.8%, 明显高于MMP-2阳性组.
细胞黏附因子(cell adhesion molecules)参与细胞的信号传导与活化, 细胞的伸展和移动, 细胞的生长和分化, 肿瘤的转移等一系列重要的生理病理过程, CD44v6为细胞黏附因子的一种, 是当前研究的热点, 我们发现CD44v6和淋巴结转移有密切关系. 本组病例中, Ⅰ期和Ⅱ期+Ⅲ期相比较, 阳性率分别为35.3%和80.8%, 两组之间差异显著(P<0.05), 也说明了CD44v6和食管鳞癌的分期相关. 本研究证实, CD44V6和血道转移相关, 在术后发生血道转移者, CD44v6的阳性表达为81.0%. 再次证实了Griffioen et al提出的CD44细胞黏附因子参与了血管形成的观点. 本组患者中CD44v6阳性的5 a生存率为6.4%, 而CD44v6阴性的患者5 a生存率为56.8%, 经过Log Rank检验P值为0.0 000有显著性差别. 说明CD44v6是影响患者预后的重要指标.
肿瘤的侵袭和转移包括一系列内在的联系的步骤, 是多分子参与的高度选择性的非随机过程. 在这个复杂过程中, 癌细胞之间, 癌细胞与基质间, 以及癌细胞与宿主细胞间的黏附至关重要. 癌细胞转移有三步骤, 即(1)癌细胞自身黏附性降低, 脱离原发部位; (2)降解, 癌细胞和宿主细胞分泌多种蛋白酶, 水解细胞外基质和基质膜中蛋白成分; (3)移动, 最后癌细胞进入淋巴管或血管向远处转移, 黏附因子与癌转移过程密切相关; 血管生成是肿瘤生长转移等复杂过程中重要环节, 我们选择CD44v6及MMP-2因子观察其在食管鳞癌中的表达, 发现CD44v6和MMP-2在食管癌中的表达是正相关, 如表2所示MMP-2表达阳性组的CD44v6阳性例数明显高于MMP-2表达阴性组的CD44v6阳性例数, 经过speraman 等级相关分析发现食管癌中MMP-2表达与CD44v6表达成正相关(r = 0.785 P<0.05)我们在生存分析中发现, CD44v6和MMP-2全部阴性的患者3 a生存率为 81.82%, 5 a生存率为72.73%, 其中一个阳性组的患者的3 a生存率38.46%, 5 a生存率为28.85%, 全部阳性组的3 a生存率为10.53%, 5 a生存率为0%, 三组之间进行检验有显著性差异(P = 0.001). 表明联合检测CD44V6和MMP-2在食管鳞癌的表达可以帮助我们判断患者的病程发展及预后, Zhang et al利用肺癌细胞株QG90研究证明CD44S能够调节MMP-2分泌. 对于二者在食管癌中的具体作用机制尚需进一步研究.
编辑: 张海宁 电编: 潘伯荣
| 1. | Ming SH, Sun TY, Xiao W, Xu XM. Matrix metalloproteinases-2, -9 and tissue inhibitor of metallo-proteinase-1 in lung cancer invasion and metastasis. Chin Med J (Engl). 2005;118:69-72. [PubMed] |
| 2. | Curran S, Dundas SR, Buxton J, Leeman MF, Ramsay R, Murray GI. Matrix metalloproteinase/tissue inhibitors of matrix metalloproteinase phenotype identifies poor prognosis colorectal cancers. Clin Cancer Res. 2004;10:8229-8234. [PubMed] |
| 3. | Schnaeker EM, Ossig R, Ludwig T, Dreier R, Oberleithner H, Wilhelmi M, Schneider SW. Microtubule-dependent matrix metalloproteinase-2/matrix metalloproteinase-9 exocytosis: prerequisite in human melanoma cell invasion. Cancer Res. 2004;64:8924-8931. [PubMed] |
| 4. | Naka T, Boltze C, Kuester D, Schulz TO, Samii A, Herold C, Ostertag H, Roessner A. Expression of matrix metallopr-oteinase (MMP)-1, MMP-2, MMP-9, cathepsin B, and urokinase plasminogen activator in non-skull base chordoma. Am J Clin Pathol. 2004;122:926-930. [PubMed] |
| 5. | Yu C, Zhou Y, Miao X, Xiong P, Tan W, Lin D. Functional haplotypes in the promoter of matrix metalloproteinase-2 predict risk of the occurrence and metastasis of esophageal cancer. Cancer Res. 2004;64:7622-7628. [PubMed] |
| 6. | Drew AF, Blick TJ, Lafleur MA, Tim EL, Robbie MJ, Rice GE, Quinn MA, Thompson EW. Correlation of tumor- and stromal-derived MT1-MMP expression with progression of human ovarian tumors in SCID mice. Gynecol Oncol. 2004;95:437-448. [PubMed] |
| 7. | Pellikainen JM, Ropponen KM, Kataja VV, Kellokoski JK, Eskelinen MJ, Kosma VM. Expression of matrix metalloproteinase (MMP)-2 and MMP-9 in breast cancer with a special reference to activator protein-2, HER2, and prognosis. Clin Cancer Res. 2004;10:7621-7628. [PubMed] |
| 8. | Lin TS, Chiou SH, Wang LS, Huang HH, Chiang SF, Shih AY, Chen YL, Chen CY, Hsu CP, Hsu NY. Expression spectra of matrix metalloproteinases in metastatic non-small cell lung cancer. Oncol Rep. 2004;12:717-723. [PubMed] |
| 9. | Ishibashi Y, Matsumoto T, Niwa M, Suzuki Y, Omura N, Hanyu N, Nakada K, Yanaga K, Yamada K, Ohkawa K. CD147 and matrix metallopro-teinase-2 protein expression as significant prognostic factors in esophageal squamous cell carcinoma. Cancer. 2004;101:1994-2000. [PubMed] |
| 10. | Sis B, Sagol O, Kupelioglu A, Sokmen S, Terzi C, Fuzun M, Ozer E, Bishop P. Prognostic significance of matrix metalloproteinase-2, cathepsin D, and tenascin-C expression in colorectal carcinoma. Pathol Res Pract. 2004;200:379-387. [PubMed] |
| 11. | Kerr KM, MacKenzie SJ, Ramasami S, Murray GI, Fyfe N, Chapman AD, Nicolson MC, King G. Expression of Fhit, cell adhesion molecules and matrix metalloproteinases in atypical adenomatous hyperplasia and pulmonary adenocarcinoma. J Pathol. 2004;203:638-644. [PubMed] |
| 12. | Miyata Y, Kanda S, Nomata K, Hayashida Y, Kanetake H. Expression of metalloproteinase-2, metalloproteinase-9, and tissue inhibitor of metalloproteinase-1 in transitional cell carcinoma of upper urinary tract: correlation with tumor stage and survival. Urology. 2004;63:602-608. [PubMed] |
| 14. | Leppa S, Saarto T, Vehmanen L, Blomqvist C, Elomaa I. A high serum matrix metalloproteinase-2 level is associated with an adverse prognosis in node-positive breast carcinoma. Clin Cancer Res. 2004;10:1057-1063. [PubMed] |
| 15. | Koyama S, Maruyama T, Adachi S. Expression of epidermal growth factor receptor and CD44 splicing variants sharing exons 6 and 9 on gastric and esophageal carcinomas: a two-color flow-cytometric analysis. J Cancer Res Clin Oncol. 1999;125:47-54. [PubMed] |
| 16. | Bottger TC, Youssef V, Dutkowski P, Maschek H, Brenner W, Junginger T. Expression of CD44 variant proteins in adenocarcinoma of Barrett's esophagus and its relation to prognosis. Cancer. 1998;83:1074-1080. [PubMed] |
| 18. | Roye GD, Myers RB, Brown D, Poczatek R, Beenken SW, Grizzle WE. CD44 expression in dysplastic epithelium and squamous-cell carcinoma of the esophagus. Int J Cancer. 1996;69:254-258. [PubMed] |
| 19. | Faleiro-Rodrigues C, Lopes C. E-cadherin, CD44 and CD44v6 in squamous intraepithelial lesions and invasive carcinomas of the uterine cervix: an immunohistochemical study. Pathobiology. 2004;71:329-336. [PubMed] |
| 20. | Bar JK, Grelewski P, Popiela A, Noga L, Rabczynski J. Type IV collagen and CD44v6 expression in benign, malignant primary and metastatic ovarian tumors: correlation with Ki-67 and p53 immunoreactivity. Gynecol Oncol. 2004;95:23-31. [PubMed] |
| 21. | Vizoso FJ, Fernandez JC, Corte MD, Bongera M, Gava R, Allende MT, Garcia-Muniz JL, Garcia-Moran M. Expression and clinical significance of CD44V5 and CD44V6 in resectable colorectal cancer. J Cancer Res Clin Oncol. 2004;130:679-686. [PubMed] |
| 22. | Kawano T, Nakamura Y, Yanoma S, Kubota A, Furukawa M, Miyagi Y, Tsukuda M. Expression of E-cadherin, and CD44s and CD44v6 and its association with prognosis in head and neck cancer. Auris Nasus Larynx. 2004;31:35-41. [PubMed] |
| 23. | Rodrigo JP, Dominguez F, Alvarez C, Herrero A, Suarez C. Expression of E-cadherin, CD44s, and CD44v6 in laryngeal and pharyngeal carcinomas. Am J Otolaryngol. 2003;24:384-389. [PubMed] |