修回日期: 2005-01-25
接受日期: 2005-02-02
在线出版日期: 2005-03-01
目的: 观察苦参碱对刀豆蛋白A(Con A)性肝损伤小鼠IFN-γ和TNF-α释放及肝组织病理改变的影响.
方法: NIH小鼠48只随机分为5组, 分别为正常对照组, 模型组, 苦参碱大剂量组(25 mg/kg), 苦参碱小剂量组(12.5 mg/kg)和联苯双酯治疗组. 除正常对照组外, 其他组于实验首日iv Con A 20 mg/kg, 苦参碱大剂量组和小剂量组均采用尾iv给药, 联苯双酯组按150 mg/kg灌胃, 每天1次, 连续3 d, 末次给药后4 h, 再次iv Con A 20 mg/kg, 8 h采血检测血浆IFN-γ和TNF-α含量、ALT活性, 观察肝组织病理学变化.
结果: 苦参碱大剂量组、小剂量组小鼠IFN-γ和TNF-α含量均明显低于模型组(IFN-γ: 25.5±6.1 vs 69.3±33.6 ng/L, 26.5±2.5 vs 69.3±33.6 ng/L, t = 4.0, 4.0, P<0.01; TNF-α: 49.1±11.9 vs 106.7±64.4 ng/L, 52.9±5.2 vs 106.7±64.4 ng/L, t = 2.9, 2.9, P<0.01), 但与联苯双酯组比较, 差异无显著性意义(P>0.05); 苦参碱大、小剂量组血浆ALT活性明显低于模型组(1 086.9±675.8 vs 2 477.2±529.9 nkat/L、1 121.9±957.4 vs 2 477.2±529.9 nkat/L, t = 5.1, 3.9, P<0.01), 且可明显减轻肝细胞坏死及炎性细胞浸润的肝组织病理学改变, 与模型组比较差异有显著性意义(P<0.05).
结论: 苦参碱对刀豆蛋白A性肝损伤小鼠释放IFN-γ和TNF-α有明显的抑制作用. 并可显著减轻肝组织病理改变.
引文著录: 李常青, 刘丽丽, 莫传伟, 黄玲. 苦参碱对Con A性肝损伤小鼠IFN释放及肝组织病理改变的影响. 世界华人消化杂志 2005; 13(5): 640-643
Revised: January 25, 2005
Accepted: February 2, 2005
Published online: March 1, 2005
AIM: To investigate the effects of matrine on the release of interferon-gamma (IFN-γ) and tumor necrosis factor alpha (TNF-α), and the hepatic histopathology in mice with concanavalin A (Con A) induced liver injury.
METHODS: Forty-eight NIH mice were randomly divided into 4 groups: control group (group A), model group (group B), big dosage group of matrine (25 mg/kg, group C), small dosage group of matrine (12.5 mg/kg, group D) and bifendate group (group E). All mice except group A were injected with Con A (20 mg/kg) via the tail vein at the first day of experiment. Group C and group D were injected with matrine (25 mg/kg and 12.5 mg/kg, respectively) via the tail vein. Mice in group E were orally administered with bifendate (150 mg/kg). All the drugs were given once daily for 3 days consecutively. Four hours after the last administration of the drugs, mice were injected with Con A once again at the same dosage. Blood samples for determining aminotransferase (ALT) activity, IFN-γ and TNF-α concentration were collected at the 8th hour after Con A administration. Histopathological examination was also performed for liver tissue.
RESULTS: Serum IFN-γ and TNF-α in group C and group D were obviously lower than that in group B(IFN-γ: 25.5±6.1 vs 69.3±33.6 ng/L, 26.5±2.5 vs 69.3±33.6 ng/L, t = 4.0, 4.0, respectively, P<0.01; TNF-α: 49.1±11.9 vs 106.7±64.4 ng/L, 52.9±5.2 vs 106.7±64.4 ng/L, t = 2.9, 2.9, respectively, P<0.01, but there was no significant difference compared with group E(P >0.05). Serum ALT activity in group C and group D were apparently lower than that of group B (1 086.9±675.8 vs 2 477.2±529.9 nkat/L, 1 121.9±957.4 vs 2 477.2±529.9 nkat/L, t = 5.1, 3.9, respectively, P<0.01). Hepatic histopathology changes were alleviated in both group C and group D compared with group E (P<0.05).
CONCLUSION: Matrine has a remarkable therapeutic effect on liver injury in mice by suppressing the release of IFN-γ and TNF-α, and alleviating the pathological changes in liver tissue.
- Citation: Li CQ, Liu LL, Mo CW, Huang L. Effects of Matrine on release of interferon and pathological changes in concanavalin A-induced liver injury in mice. Shijie Huaren Xiaohua Zazhi 2005; 13(5): 640-643
- URL: https://www.wjgnet.com/1009-3079/full/v13/i5/640.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v13.i5.640
苦参碱为中药苦参、山豆根和苦豆子的主要有效成分, 苦参碱对多种实验性肝损伤模型(包括CCl4、脂多糖/D-氨基半乳糖、猪血清)均有肝细胞保护作用[1-4], 并可抑制Kupffer细胞、巨噬细胞释放炎性因子TNF和IL-6[3-5], 但尚未见到苦参碱对刀豆蛋白A(Con A)性肝损伤模型影响的研究报道. 我们以NIH小鼠为实验对象, 探讨苦参碱对Con A性肝损伤小鼠IFN-γ和TNF-α释放的影响, 并观察肝脏病理组织学改变.
NIH♂小鼠, 体18-22 g, 广东省医学实验动物中心提供, 刀豆蛋白A为美国华盛顿生物药品公司产品; 苦参碱注射液, 广州明兴制药厂生产, 每支5 mL含苦参碱50 mg; 联苯双酯滴丸, 每丸含联苯双酯7.5 mg. 谷丙转氨酶检测试剂盒购自上海申索试剂有限公司, 小鼠干扰素γ(IFN-γ)和肿瘤坏死因子(TNF-α)定量检测试剂盒购自上海森雄科技实业有限公司, 752型紫外分光光度计(上海第三分析仪器厂), Bio-Tek ELX8000自动酶标仪(Bio-Tek instrument INC).
NIH小鼠48只随机分为5组: 正常对照组8只, 模型组、苦参碱大剂量组(25 mg/kg)、苦参碱小剂量组(12.5 mg/kg)和联苯双酯治疗组各10只, 除正常对照组外, 其余小鼠于实验首日上午尾iv Con A 20 mg/kg, 并于首日、次日和第3 d下午各给药1次, 苦参碱大剂量组、小剂量组(相当于10倍、5倍临床用药剂量)均采用尾iv给药, 联苯双酯按150 mg/kg每日灌胃, 末次给药后4 h, 模型组和各给药组小鼠1次性iv Con A 20 mg/kg, 禁食, 不禁水, 8 h摘小鼠眼球取血, 离心分离血浆待检; 取肝左叶组织, 40 g/L甲醛溶液固定. 血浆IFN-γ和TNF-α定量测定采用双抗体夹心ABC-ELISA法, 按试剂盒说明操作, 用Bio-Tek ELX8000自动酶标仪于波长490 nm处检测吸光度值, 制定标准曲线, 换算成IFN-γ和TNF-α定量值. 血浆ALT活性检测采用赖氏法, 按试剂盒说明操作, 用752型紫外分光光度计于波长505 nm处检测吸光度值, 制定标准曲线, 换算成ALT值. 肝组织石蜡包埋切片, 常规HE染色后行光镜病理观察. 组织病变分级参照文献[6], 肝细胞坏死分级: 0级: 无病变; Ⅰ级: 肝细胞疏松肿胀, 气球样变, 有点状坏死.Ⅱ级: 肝细胞灶性坏死.Ⅲ级: 肝细胞广泛灶性坏死. 炎性细胞浸润分级: 0级: 汇管区极少炎性细胞浸润.Ⅰ级: 汇管区、血管窦可见多个炎性细胞浸润.Ⅱ级: 汇管区、肝小叶内炎性细胞浸润明显, 肝实质破坏.Ⅲ级: 汇管区大量炎性细胞浸润, 肝实质破坏.
统计学处理 采用SPSS10.0软件包进行统计分析, 多组计量资料分析采用单因素方差分析(One-way ANOVA), 多重比较采用最小显著差值法(LSD); 多组等级资料的分析采用Kruskal-Wallis检验, 两两比较采用Nemenyi法.
模型组小鼠IFN-γ, TNF-α含量和ALT活性较正常对照组明显升高, 二者比较差异有显著性意义(t = 5.3, 3.4, 10.8, P<0.01, 表1). 苦参碱大剂量组、小剂量组小鼠IFN-γ, TNF-α含量和ALT活性均明显低于模型组, 差异有显著性意义(t = 4.0, 4.0, 2.9, 2.9, 5.1, 3.9, P<0.01), 但与联苯双酯组比较, 差异无显著性意义(t = 1.4, 1.9, 1.5, 1.1, 1.4, 0.1, P>0.05). 苦参碱大剂量组与小剂量组比较, 小鼠IFN-γ, TNF-α含量和ALT活性的差异无显著性意义(t = 0.4, 0.9, 0.1, P>0.05).
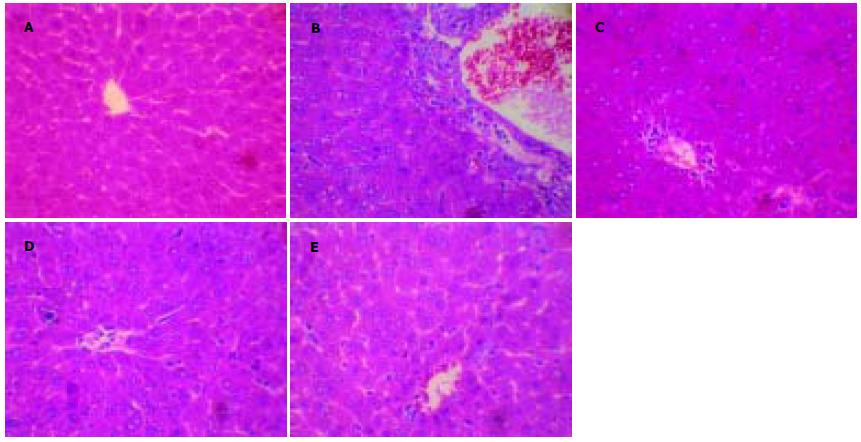
光镜下见正常组肝细胞以中央静脉为中心呈放射状排列, 肝窦未见异常. 模型组全部小鼠肝组织均出现明显病变, 肝小叶内大多数肝细胞肿胀, 细胞质疏松化, 有的呈气球样变, 可见凋亡小体、明显的点状坏死和灶性坏死, 坏死灶可见大量炎性细胞浸润, 汇管区淋巴细胞和单核细胞炎性浸润尤为明显, 肝窦内可见红细胞堆集. 苦参碱大剂量、小剂量组和联苯双酯治疗组部分肝组织可见散在点状坏死和灶性坏死, 但肝细胞坏死程度与模型组相比明显减轻, 炎性细胞浸润显著减少. 采用Kruskal-Wallis法进行检验, 结果显示组间肝细胞坏死、炎性细胞浸润的病理学改变差异均有显著性意义(Hc = 22.29, 26.64, P<0.01), 采用Nemenyi法两两比较, 结果显示苦参碱大、小剂量组和联苯双酯组的肝细胞坏死、炎性细胞浸润病理改变均明显低于模型组, 差异具有显著性意义(D = 18.50, 17.55, 17.55, 20.30, 19.40, 20.30, P<0.05, 表2, 图1).
| 分组 | n | 肝细胞坏死 | 炎性细胞浸润 | ||||||
| 0级 | Ⅰ级 | Ⅱ级 | Ⅲ级 | 0级 | Ⅰ级 | Ⅱ级 | Ⅲ级 | ||
| 正常组 | 8 | 8 | 0 | 0 | 0 | 8 | 0 | 0 | 0 |
| 模型组 | 10 | 0 | 1 | 6 | 3 | 0 | 0 | 3 | 7 |
| 苦参碱大剂量 | 10 | 6 | 2 | 2 | 0 | 5 | 2 | 3 | 0 |
| 苦参碱小剂量 | 10 | 6 | 1 | 3 | 0 | 5 | 1 | 4 | 0 |
| 联苯双酯组 | 10 | 6 | 1 | 3 | 0 | 5 | 2 | 3 | 0 |
Con A是一种被广泛应用可活化T细胞的有丝分裂原, Con A诱发的小鼠肝损伤是近年来建立的实验动物模型, 通过活化T淋巴细胞而致免疫性肝损伤是该动物模型的基本病理特点, 与其他中毒性肝损伤模型如四氯化碳模型、D-氨基半乳糖模型相比, 该模型被认为更适于研究人类病毒性肝炎、自身免疫性肝病等的病理机制和进行抗免疫性肝损伤的药物筛选[7-19]. Con A iv小鼠体内后, 大部分在肝脏内聚集, 表明肝脏是Con A体内诱导毒性的靶器官[7]. 肝窦内大量存在的巨噬细胞, 激活后产生的细胞因子TNF-α可直接损伤肝细胞, 导致肝细胞凋亡、坏死[20-23]; 其次, 大量活化的T淋巴细胞与细胞因子IFN-γ等随血流到达肝脏, 直接与肝细胞接触或进一步激活巨噬细胞、破坏血管内皮细胞导致肝损伤[24-25]. 细胞因子IFN-γ、TNF-α是引起急性肝损伤的重要炎症递质[26-27], T淋巴细胞活化后产生的IFN-γ是巨噬细胞的重要激活剂, 对促进肝内Kupffer细胞参与炎症反应和刺激巨噬细胞分泌TNF-α起作重要作用[28-30], 研究表明, 预先给予抗-IFN-γ, 或抗-TNF-α, 均可完全阻断Con A诱发的小鼠肝损伤[7,31].
本结果显示, 3 d内2次iv Con A20 mg/kg, 模型组小鼠血浆IFN-γ, TNF-α含量增加, ALT活性升高; 病理学检查发现, Con A组小鼠肝组织汇管区内大量炎性细胞浸润, 且有点状和灶性坏死. 该结果再次证实T淋巴细胞活化导致IFN-γ分泌增加及刺激巨噬细胞释放过多TNF-α是Con A性肝损伤的主要病理机制. 苦参碱2个剂量组Con A诱发的急性肝损伤小鼠IFN-γ和TNF-α含量明显降低, 肝细胞坏死、炎性细胞浸润的病理组织学改变显著减轻, 谷丙转氨酶活性降低, 表明苦参碱可通过抑制T淋巴细胞活化和IFN-γ, TNF-α的释放发挥抗肝损伤作用. 但苦参碱大剂量组和小剂量组对小鼠IFN-γ, TNF-α的含量和肝组织病理组织学改变的差异无显著性意义(P>0.05), 提示超过一定的用药剂量, 并不能明显提高苦参碱抗肝损伤的作用效果, 与文献[4]报道基本一致, 因此, 选择合适的剂量用药, 或进行剂型改造, 如制备成肝靶向制剂等以提高其作用效果, 均有待进一步深入研究.
编辑: 张海宁 电编: 潘伯荣
| 5. | Zhu XH, Qiu YD, Shen H, Shi MK, Ding YT. Effect of matrine on Kupffer cell activation in cold ischemia reperfusion injury of rat liver. World J Gastroenterol. 2002;8:1112-1116. [PubMed] |
| 7. | Tiegs G, Hentschel J, Wendel A. A T cell-dependent experimental liver injury in mice inducible by Concanavalin A. J Clin Invest. 1992;90:196-203. [PubMed] |
| 10. | Khakoo SI, Ling R, Scott I, Dodi AI, Harrison TJ, Dusheiko GM, Madrigal JA. Cytotoxic T lymphocyte responses and CTL epitope escape mutation in HBsAg, anti-HBe positive individuals. Gut. 2000;47:137-143. [PubMed] |
| 13. | Rehermann B. Intrahepatic T cells in hepatitis B: viral control versus liver cell injury. J Exp Med. 2000;191:1263-1268. [PubMed] |
| 15. | 王 九平, 李 新红, 朱 勇, 王 爱莲, 连 建奇, 贾 战生, 谢 玉梅. 慢性乙型肝炎患者T细胞亚群, MIL-2R, sIL-2R, IL-6, IL-8, TNF-α变化及意义. 世界华人消化杂志. 2000;8:763-766. [DOI] |
| 16. | Wang JH, Layden TJ, Eckels DD. Modulation of the peripheral T-Cell response by CD4 mutants of hepatitis C virus: transition from a Th1 to a Th2 response. Hum Immunol. 2003;64:662-673. [PubMed] |
| 18. | Miyazawa Y, Tsutsui H, Mizuhara H, Fujiwara H, Kaneda K. Involvement of intrasinusoidal hemostasis in the development of concanavalin A-induced hepatic injury in mice. Hepatology. 1998;27:497-506. [PubMed] |
| 20. | 李 小刚, 王 天才, 武 忠弼, 周 开敏, 唐 望先, 李 绍白. 刀豆蛋白A诱导急性肝损伤的病理学观察. 临床与实验与病理学杂志. 1998;14:492-493. |
| 21. | Gantner F, Leist M, Lohse AW, Germann PG, Tiegs G. Concanavalin A induced T-cell-mediated hepatic injury in mice: the role of tumor necrosis factor. Hepatology. 1995;21:190-198. [PubMed] |
| 22. | Ksontini R, Colagiovanni DB, Josephs MD, Edwards CK 3rd, Tannahill CL, Solorzano CC, Norman J, Denham W, Clare-Salzler M, MacKay SL, Moldawer LL. Disparate roles for TNF-alpha and Fas ligand in concanavalin A-induced hepatitis. J Immunol. 1998;160:4082-4089. [PubMed] |
| 24. | Kusters S, Gantner F, Kunstle G, Tiegs G. Interferon gamma plays a critical role in T cell-dependent liver injury in mice initiated by Concanavalin A. Gastroenterology. 1996;111:462-471. |
| 25. | Knolle PA, Gerken G, Loser E, Dienes HP, Gantner F, Tiegs G, Meyer zum Buschenfelde KH, Lohse AW. Role of sinusoidal endothelial cells of the liver in concanavalin A-induced hepatic injury in mice. Hepatology. 1996;24:824-829. [PubMed] |
| 26. | Tiegs G. Experimental hepatitis and role of cytokines. Acta Gastroenterol Belg. 1997;60:176-179. [PubMed] |
| 29. | Jaruga B, Hong F, Kim WH, Gao B. IFN-gamma/STAT1 acts as a proinflammatory signal in T cell-mediated hepatitis via induction of multiple chemokines and adhesion molecules: a critical role of IRF-1. Am J Physiol Gastrointest Liver Physiol. 2004;287:1044-1052. [PubMed] |
| 30. | Morita A, Itoh Y, Toyama T, Fujii H, Nishioji K, Kirishima T, Makiyama A, Yamauchi N, Okanoue T. Activated Kupffer cells play an important role in intra-hepatic Th1-associated necro-inflammation in Concanavalin A-induced hepatic injury in mice. Hepatol Res. 2003;27:143-150. [PubMed] |