修回日期: 2005-01-15
接受日期: 2005-01-20
在线出版日期: 2005-03-01
目的: 研究热休克肿瘤细胞抗原负载的树突状细胞的体内抗肿瘤作用以及对原位接种结肠肿瘤小鼠的免疫保护作用.
方法: 肿瘤细胞CT-26经热休克处理后制备冻融抗原, 体外负载树突状细胞, 免疫同源小鼠(热休克组). 以IFN-γ ELISPOT实验、LDH释放实验检测小鼠体内肿瘤特异性T细胞免疫反应, 以原位接种结肠肿瘤的体积以及荷瘤小鼠的生存时间来评价该树突状细胞瘤苗对结肠原位接种CT-26小鼠的免疫保护作用, 并与非热休克肿瘤细胞抗原负载的树突状细胞的免疫效果(非热休克组)作一比较.
结果: 热休克处理肿瘤细胞可提高肿瘤细胞抗原负载的树突状细胞表面MHC-Ⅱ类分子(66.3% vs 59.1%)、共刺激分子CD86的表达(39.4% vs 36.7%); 热休克组小鼠脾淋巴细胞中肿瘤特异性CTL数量高于非热休克组(P = 0.001), 相同效靶比下前者肿瘤特异性细胞毒活性强于后者; 热休克组与非热休克组小鼠原位接种结肠肿瘤14 d后的肿瘤体积均明显小于单纯DC免疫组(P = 0.000), 但HSCT-26DC免疫组与CT-26 DC免疫组间差异无显著性(P = 0.480). 单纯DC免疫组50%(3/6)可见腹膜转移, CT-26 DC免疫组以及HSCT-26DC免疫组均未见腹腔转移; 热休克组小鼠荷瘤生存时间显著长于非热休克组(P = 0.0384).
结论: 热休克处理肿瘤细胞可以提高肿瘤细胞抗原负载的树突状细胞的体内抗肿瘤作用, 热休克肿瘤细胞抗原负载的树突状细胞瘤苗对原位接种结肠肿瘤小鼠具有良好的免疫保护作用.
引文著录: 邱健, 李国威, 隋延仿, 宋宏萍, 司少艳, 葛伟. 热休克肿瘤细胞抗原负载的树突状细胞瘤苗对原位接种结肠肿瘤的免疫预防作用. 世界华人消化杂志 2005; 13(5): 635-639
Revised: January 15, 2005
Accepted: January 20, 2005
Published online: March 1, 2005
AIM: To investigate the antitumor effect of dendritic cells (DC) pulsed with lysates of heat-shocked tumor cells and the prophylactic effects of this DC tumor vaccine in mice inoculated with tumor cells in colon.
METHODS: CT-26 cells were heat-shocked at 42℃ for 1 h (HSCT-26) and then frozen-thawed repeatedly to lyse. Bone marrow-derived DCs pulsed with the lysate were used to immunize BALB/c mice. Using IFN-γ enzyme-linked immunospot (ELISPOT) and LDH release assay, the anti-tumor response of cytotoxic T lymphocytes (CTLs) was evaluated. The immunoprophylactic effect induced by heat-shocked CT-26 cell lysate pulsed DC (HSCT-26 DC) in colon cancer mouse model was compared with that induced by non-heat-shocked CT-26 cells lysate pulsed DC (CT-26 DC) on tumor volume and survival time.
RESULTS: Compared with CT-26 lysates, pulsing with HSCT-26 lysate enhanced the expression of MHC-II molecule (66.3% vs 59.1%) and co-stimulating molecule CD86 (39.4% vs 36.7%) on DC surface. The quantity of CTLs induced by HSCT-26 DC was more than that induced by CT-26 DC (P = 0.001). The specific cytotoxic activity of CTLs induced by HSCT-26 DC was more potent than that of CTLs induced by CT-26 DC at the same E: T ratio. Fourteen days after colon inoculation of CT-26 cells, the tumor volume of HSCT-26 DC immunized mice was similar to that of CT-26 DC immunized mice (P = 0.480), and both were much smaller than that of DC immunized mice (P = 0.000). 50% of DC immunized mice suffered from peritoneal metastasis, whereas none of HSCT-26 DC immunized mice and CT-26 DC immunized mice was involved. The survival time of colon cancer model mice immunized with HSCT-26 DC was significantly longer than that of the mice immunized with CT-26 DC (P = 0.0384).
CONCLUSION: Pre-heated tumor cells can enhance the antitumor effects elicited by tumor cell lysate pulsed DCs in vivo. Vaccination with DCs pulsed with lysates of heat-shocked tumor cells can prolong the survival time of mice with colon cancer by effectively suppressing the growth of colon cancer and peritoneal metastasis.
- Citation: Qiu J, Li GW, Sui YF, Song HP, Si SY, Ge W. Prophylactic effects of dendritic cells pulsed with lysates of heat-shocked tumor cells in mice with colonic inoculation of tumor cells. Shijie Huaren Xiaohua Zazhi 2005; 13(5): 635-639
- URL: https://www.wjgnet.com/1009-3079/full/v13/i5/635.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v13.i5.635
结直肠癌是我国人群多发肿瘤之一, 近年来其发病率有逐渐上升的趋势[1]. 目前结直肠癌治疗首选手术切除, 但是对于术后复发或转移尚缺乏有效的治疗手段[2-3]. 肿瘤细胞冻融抗原负载的树突状细胞(Dendritic cell, DC), 可以诱导针对该抗原的特异性细胞毒性T淋巴细胞, 发挥有效的抗肿瘤免疫效应[4-6]. 如何进一步增强肿瘤细胞抗原负载的DC的抗肿瘤免疫效应, 有待深入研究. 近年来, 有研究显示热休克处理能够增强肿瘤细胞的免疫原性, 提高肿瘤细胞裂解产物的免疫反应[7-9]. 我们探讨热休克处理肿瘤细胞能否提高肿瘤抗原致敏DC的抗肿瘤免疫效应以及该DC瘤苗对原位接种结肠肿瘤小鼠的免疫保护作用.
RPMI 1640, AIM-V, DMEM培养基为Gibco产品, Ficoll淋巴细胞分离液购自鼎国生物技术公司, 胎牛血清(超级)购自杭州四季青生物制品公司, 重组小鼠粒-巨细胞集落刺激因子(rmGM-CSF)为R&D生物工程公司产品, FITC-仓鼠抗小鼠 CD11c mAb, PE-大鼠抗小鼠 I-A/I-E mAb, CD86 mAb及其同型对照为Biolegend公司产品, 购自晶美生物公司. 小鼠IFN-γ Elispot检测试剂盒为Diaclone产品, Cytotox 96检测试剂盒为Promega公司产品. 恒温CO2培养箱(3548型)产自Forma Scientific公司, 倒置显微镜为日本Olympus公司产品, 流式细胞仪FACS calibur为美国BD 公司产品.6-8 wk雌性BALB/c小鼠(H-2 d)购自第四军医大学实验动物中心, 饲养于SPF级环境; BALB/c鼠源结肠未分化腺癌细胞株CT-26(ATCC CRL2638)购自中南大学湘雅医学院肿瘤研究所, 培养于含100 mL/LFBS DMEM, 每3 d传代.
1.2.1 小鼠骨髓源树突状细胞的体外诱导培养及表型鉴定: 参考Lutz et al[10]方法, 小鼠脱颈处死, 750 mL/L酒精浸泡10 min, 取胫骨与股骨, 750 mL/L酒精浸泡1 min, RPMI1640冲洗, 剪去胫、股骨两端, 取2 mL RPMI1640冲洗髓腔, 洗液重复吸打后过200目钢网, 1 000 r/min离心10 min, 红细胞裂解液10 mL重悬, 4℃静置5 min, 1 000 r/min离心10 min, 完全培养基(RPMI1640含100 mL/L FBS、100 U/mL青霉素、100 U/mL链霉素、10 mmol/L HEPES、50 mmol/L丙酮酸钠、20 μg/L rmGM-CSF)重悬, 1×109/L接种10 cm培养皿.3 d半量更换完全培养液. 收获的DC作表面分子CD11c、I-A/I-E、CD86荧光直标抗体染色, 流式细胞仪检测.
1.2.2 热休克处理肿瘤细胞及其冻融抗原的制备并负载树突状细胞: CT-26细胞90%融合时, 置42℃恒温水浴热休克1 h, 37℃, 50 mL/LCO2恢复2 h, 胰酶消化, 无血清RPMI1640洗涤后重悬, 调整细胞密度为1×109/L, 液氮速冻10 min, 4℃融化, 反复3次, 12 000 g离心10 min, 取上清为热休克肿瘤细胞(HSCT-26)冻融抗原; 同时取未经热休克的CT-26细胞制备冻融抗原. 收获第7 d培养DC, 完全培养基调整细胞数密度为1×109/L, 种6孔板5 mL/孔. 实验组接种HSCT-26冻融抗原1 mL, 对照组接种CT-26冻融抗原1 mL, 阴性对照组接种无血清RPMI1640 1 mL.37℃, 50 mL/L CO2孵育48 h, 收获DC, PBS洗涤后重悬, 调整DC密度为5×109/L.
1.2.3 小鼠的免疫与结肠肿瘤原位接种方法: BALB/c小鼠9只, 随机分入3组, 每组3只. 第1 d实验组尾静脉注射HSCT-26 DC 5×105(100 μL)/只[11], 对照组注射CT-26 DC 5×105(100 μL)/只, 阴性对照组注射未致敏DC 5×105(100 μL)/只, 第7 d同法再次免疫, 第14 d处死检测脾淋巴细胞肿瘤特异性CTL的数量及细胞毒活性. 另BALB/c小鼠36只, 免疫方法同前, 第14 d小鼠全麻下行剖腹手术, 于盲肠壁浆膜下原位注射CT-26细胞1×105(50 μL)/只, 随机分入3组, 每组12只, 第28 d每组5只处死, 解剖测量接种肿瘤的长径(L)与短径(S), 按公式V = (L碨2)/2计算肿瘤体积, 并记录转移情况. 每组剩余7只观测荷瘤生存期, 以任意两组小鼠全部死亡为观察终点.
1.2.4 IFN-γ 酶联免疫斑点实验: (IFN-γ ELISPOT)灭菌磨砂玻片研磨鼠脾, RPMI1640 3 mL重悬脾细胞并吸打数次, 淋巴细胞分离液密度梯度离心分离脾淋巴细胞, AIM-V重悬, 调整细胞密度为2×1010/L, 为效应细胞. 消化CT-26细胞, 以含50 mg/L 丝裂霉素的DMEM重悬, 37℃, 50 mL/L CO2孵育30 min, PBS洗涤3次, AIM-V重悬, 调整细胞密度为1×109/L, 为靶细胞. 按照试剂盒说明: 700 mL/L酒精洗板10 min, PBS洗3次, 甩干; PBS 10 mL稀释捕获抗体100 μL包板4℃过夜, PBS洗3次, 甩干; 20 g/L脱脂乳37℃封闭2 h, PBS洗1次, 甩干; 每孔分别加入效应细胞1×106(50 μL)、靶细胞5×104(50 μL), 37℃、50 mL/L CO2孵育20 h; 加100 μL PBS-1 g/L Tween-20(PBS-T), 4℃ 10 min, 甩干; PBS-T洗板3次, 拍干; 10 mL含10 g/LBSA PBS稀释100 μL检测抗体, 100 μL/孔, 37℃孵育90 min, 甩干, PBS-T洗板3次, 拍干; 10 mL含10 g/L BSA PBS稀释10 μL链霉亲和素-碱性磷酸酶, 100 μL/孔, 37℃孵育60 min, 甩干, PBS-T洗3次, 拍干. 加BCIP/NTB 100 μL, 室温避光显色, 观察斑点形成后蒸馏水洗板3次, 甩干后4℃过夜, 扫描后计数每孔斑点数, 取3复孔的平均值. 以斑点数代表分泌IFN-γ的肿瘤特异性CTL的数量. PHA刺激作为阳性对照, 效应细胞孔不加靶细胞为空白对照.
1.2.5 CTL的肿瘤特异性杀伤活性测定(LDH释放法): 按操作说明接种细胞至96孔圆底板: 靶细胞自发释放孔接种靶细胞5×104个, 3复孔; 靶细胞最大释放孔细胞数5×104个, 3复孔; 体积校正孔与培养液背景孔各加入AIM-V 150 μL. 效应细胞自发释放孔分别加入效应细胞2.5×105、5×105、1×106和2×106个; 实验孔分别加入2.5×105、5×105、1×106和2×106个效应细胞和5×104个靶细胞; 以AIM-V调整每孔体积为150 μL.1 000 r/min离心5 min.37℃、50 mL/L CO2孵育4 h后, 体积校正孔和靶细胞最大释放孔各加入10裂解液15 μL, 继续培养45 min.1 000 r/min离心4 min. 取每孔上清50 μL入96孔平底板. 每孔加入显色底物50 μL, 避光室温显色30 min. 加入终止液50 μL, 490 nm检测每孔光密度(Optical density, OD)值. (各孔A值的修正: 实验孔、靶细胞自发释放孔、效应细胞自发释放孔A值均减去培养液背景孔A值. 靶细胞最大释放孔A值减去体积校正孔A值.)
杀伤率(%) = (实验组A-效应细胞自发A-靶细胞自发A)/( 靶细胞最大A-靶细胞自发A)100%
统计学处理 计量资料数据以mean±SD表示, 多组间计量资料的比较采用单因素方差分析, Kaplan-Meier法绘制不同组别生存曲线, Log-rank时序检验比较生存时间, 以P<0.05为检验标准, 以SPSS8.0统计软件进行数据分析.
第7 d DC培养体系收获细胞中CD11c+ IA/IE+ 31.2%, CD11c+ CD86+ 17.7%; CT-26抗原负载后CD11c+ IA/IE+ 59.1%, CD11c+ CD86+ 36.7%; HSCT-26抗原负载后CD11c+ IA/IE+ 66.3%, CD11c+ CD86+ 39.4%; 1mg/L LPS(阳性对照)刺激24 h后CD11c+ IA/IE+ 69.8%, CD11c+CD86+45.4%; 第9 d DC培养体系收获细胞(未负载抗原)CD11c+ IA/IE+ 49.9%、CD11c+CD86+28.6%.
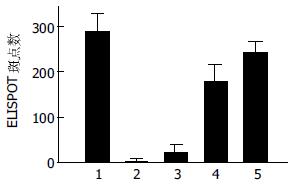
以ELISPOT方法检测1×106鼠脾淋巴细胞肿瘤特异性CTL数量, HSCT-26 DC免疫组、CT-26 DC免疫组、单纯DC免疫组间IFN-γ(+)斑点数有显著性差异(P = 0.0 001); 两两比较, HSCT-26组IFN-γ(+)斑点数明显多于CT-26组(P = 0.001, 图1).
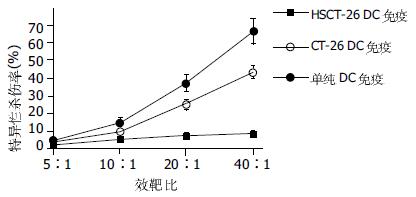
鼠脾淋巴细胞肿瘤特异性CTL的细胞毒随效靶比的上升而逐渐提高. HSCT-26 DC免疫组在效靶比为10: 1, 20: 1, 40: 1时肿瘤细胞杀伤率分别为: 14.5%, 36.9%, 66.1%, 而CT-26 DC免疫组分别为: 9.4%、25.0%、43.3%, 前者均高于后者. 各组别特异性杀伤活性曲线(图2).
单纯DC免疫组、CT-26 DC免疫组均可见盲肠壁原位肿瘤形成, HSCT-26DC免疫组5/6可见盲肠壁原位肿瘤形成, 3组肿瘤体积分别为: 107±69 mm3、24±8 mm3、8±7 mm3; HSCT-26DC免疫组以及CT-26 DC免疫组分别与DC免疫组有显著差异(P = 0.000), 但HSCT-26DC免疫组与CT-26 DC免疫组间差异无显著性(P = 0.480). DC免疫组50%(3/6)可见邻近肠道浆膜以及腹膜的种植, 伴有少量血性腹水, 未见肝脏转移. CT-26 DC免疫组以及HSCT-26DC免疫组均未见腹腔或肝脏转移.
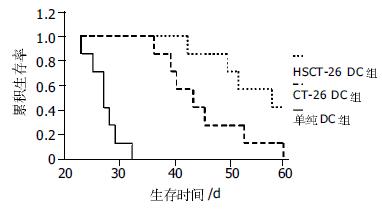
单纯DC免疫组、CT-26 DC免疫组、HSCT-26DC免疫组小鼠中位生存时间分别为: 27.0±1.3(24.4-29.6)d、43.0±3.9(35.3-50.7)d、57.0±7.9(41.6-72.4)d. Log-rank时序检验表明3组生存时间不同(P = 0.0001). CT-26 DC免疫组生存时间长于DC免疫组(P = 0.0001), 而HSCT-26DC免疫组生存时间长于CT-26 DC免疫组(P = 0.0384, 图3).
树突状细胞(Dendritic cell, DC)是体内专职的抗原递呈细胞(Antigen presentation cell, APC), 他摄取、处理肿瘤相关抗原, 以MHC-Ⅰ类分子或MHC-Ⅱ类分子依赖方式递呈至DC表面, 在共刺激分子的协同下, 激活T淋巴细胞发挥特异性的抗肿瘤免疫效应[12-13]. 目前, 激活肿瘤特异性DC的手段包括肿瘤抗原肽/蛋白负载、肿瘤抗原基因修饰、肿瘤整细胞或肿瘤细胞裂解产物刺激、肿瘤细胞与DC融合等[14]. 肿瘤整细胞或肿瘤细胞裂解产物包含一整套肿瘤抗原, 以其刺激DC可以简便有效地获得针对原发肿瘤多种抗原的多价DC瘤苗.1998年, Fields et al[15]报告肿瘤裂解产物负载DC可诱导肿瘤特异性CTL, 以其免疫小鼠可以产生针对该肿瘤的免疫预防作用. 肿瘤裂解产物负载DC瘤苗现已试用于包括黑色素瘤、肾癌、淋巴瘤、结直肠癌在内的多种肿瘤的免疫治疗[16-20]. 人们试用GM-CSF, IL-12 或 IFN-α等细胞因子基因修饰[21-22], 或联合使用免疫佐剂KLH、Flt3配体或CpG寡核苷酸等[23-24], 希望进一步提高DC瘤苗的抗肿瘤免疫效应. 热休克蛋白作为重要的分子伴侣, 与细胞内抗原肽结合, 将细胞抗原"指纹"递呈给APC[25]. 从肿瘤细胞分离的热休克蛋白可以诱导肿瘤特异性免疫反应[26], 热休克蛋白在体外与肿瘤抗原肽结合可诱导肿瘤特异性免疫反应[27], 热休克处理能够提高肿瘤细胞的抗原性, 诱导机体抗肿瘤免疫反应[7]. 通过热休克肿瘤细胞提高热休克蛋白的表达, 将其裂解产物负载DC, 如果能够高效地诱导体内抗肿瘤免疫反应, 可以简化烦琐的体外操作, 为临床提供实践性很强的制备DC瘤苗的方法.
本研究提示, 热休克处理能够提高肿瘤细胞冻融抗原负载的树突状细胞表面MHCⅡ类分子、共刺激分子的表达, 促进DC的成熟, 从而提高了DC的抗原递呈能力. 与肿瘤细胞冻融抗原负载的DC相比较, 以热休克肿瘤细胞冻融抗原负载的DC免疫小鼠, 显著增加小鼠脾脏淋巴细胞中肿瘤特异性CTL的数量并且提高CTL的细胞毒活性, 表明热休克肿瘤细胞冻融抗原负载的DC能够在体内高效地诱导抗肿瘤免疫反应. 针对原位注射肿瘤细胞建立的实验性结肠癌动物模型, 以热休克肿瘤细胞冻融抗原负载的DC免疫可以抑制肿瘤的生长与转移, 虽然接种肿瘤早期与肿瘤细胞冻融抗原负载的DC的免疫预防效果相近, 但前者显著延长荷瘤小鼠的生存时间. 这一结果提示, 热休克肿瘤细胞冻融抗原负载的DC免疫效果可能更为持久, 从而对结肠癌模型小鼠产生更好的免疫保护作用. 这与国外近期的热应激诱导凋亡肿瘤细胞致敏DC的报道相近[28]. 热休克处理提高肿瘤细胞抗原负载的DC的抗肿瘤作用的机制有待进一步阐明, 其可能的机制包括: (1)适当的热休克处理能够提高肿瘤细胞热休克蛋白的表达水平. 与肿瘤抗原结合的热休克蛋白, 能够通过DC表面分子CD40以及CD91介导而内化外源性抗原, 协助其转运、降解以及抗原肽与MHC分子的有效结合, 并递呈至DC表面, 进而激活CTL[29-30]; (2)与肿瘤细胞抗原结合的热休克蛋白可以提供危险信号(Danger signal), 促进MHCⅡ类分子、共刺激分子的表达以及细胞因子如IL-12的合成与分泌, 从而增强DC的抗原递呈能力[31-32].
临床上能够接受手术治疗的结直肠癌患者, 多为Dukes'C期以下, 可以较为完整地切除肿瘤及其局属淋巴组织, 减轻机体肿瘤负荷, 使免疫系统得以恢复. 此时辅助免疫治疗, 对于预防术后肿瘤复发、转移有重要的价值. 热休克肿瘤细胞抗原负载的DC瘤苗能够有效抑制原位结肠肿瘤的生长、减少接种肿瘤的腹腔转移灶的形成并最终延长荷瘤动物的生存时间. 本研究为临床上通过热休克肿瘤组织制备DC瘤苗进行免疫预防结直肠癌术后复发、转移提供了一定的理论依据. 该DC瘤苗对结直肠癌肝脏转移瘤的预防价值有待进一步的实验研究.
编辑: 张海宁 电编: 潘伯荣
| 1. | Chen KX, He M, Dong SF, Wang JF, Song FJ, Wu GL. The prevalence data of colon cancer in TianJin in two decades. Chin J Prev Med. 2004;38:356-357. [PubMed] |
| 2. | Nicum S, Midgley R, Kerr D. Colorectal cancer. Acta Oncol. 2003;42:263-275. [PubMed] |
| 3. | Liu LX, Zhang WH, Jiang HC. Current treatment for liver metastases from colorectal cancer. World J Gastroenterol. 2003;9:193-200. [PubMed] |
| 4. | Nestle FO, Banchereau J, Hart D. Dendritic cells: on the move from bench to bedside. Nat Med. 2001;7:761-765. [PubMed] |
| 5. | Tang ZH, Qiu WH, Wu GS, Yang XP, Zou SQ, Qiu FZ. The immunotherapeutic effect of dendritic cells vaccine modified with interleukin-18 gene and tumor cell lysate on mice with pancreatic carcinoma. World J Gastroenterol. 2002;8:908-912. [PubMed] |
| 6. | Xie YA, Luo XL, Liang AM, Kuang ZP, Wu JN. Hepatic cancer antigen primed dendritic cells induces specific cytotoxic T lymphocytes in vitro. Shijie Huaren Xiaohua Zazhi. 2004;12:1479-1481. |
| 7. | Okamoto M, Tazawa K, Kawagoshi T, Maeda M, Honda T, Sakamoto T, Tsukada K. The combined effect against colon-26 cells of heat treatment and immunization with heat treated colon-26 tumor cell extract. Int J Hyperthermia. 2000;16:263-273. [PubMed] |
| 8. | Clark PR, Menoret A. The inducible Hsp70 as a marker of tumor immunogenicity. Cell Stress Chaperones. 2001;6:121-125. [PubMed] |
| 9. | Todryk SM, Eaton J, Birchall L, Greenhalgh R, Soars D, Dalgleish AG, Melcher AA, Pandha HS. Heated tumour cells of autologous and allogeneic origin elicit anti-tumor immunity. Cancer Immunol Immunother. 2004;53:323-330. [PubMed] |
| 10. | Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77-92. [PubMed] |
| 11. | Son YI, Egawa S, Tatsumi T, Redlinger RE Jr, Kalinski P, Kanto T. A novel bulk-culture method for generating mature dendritic cells from mouse bone marrow cells. J Immunol Methods. 2002;262:145-157. [PubMed] |
| 12. | Banchereau J, Schuler-Thurner B, Palucka AK, Schuler G. Dendritic cells as vectors for therapy. Cell. 2001;106:271-274. [PubMed] |
| 13. | Steinman RM, Dhodapkar M. Active immunization against cancer with dendritic cells: the near future. Int J Cancer. 2001;94:459-473. [PubMed] |
| 14. | Schuler G, Schuler-Thurner B, Steinman RM. The use of dendritic cells in cancer immunotherapy. Curr Opin Immunol. 2003;15:138147. [PubMed] |
| 15. | Fields RC, Shimizu K, Mule JJ. Murine dendritic cells pulsed with whole tumor lysates mediate potent antitumor immune responses in vitro and in vivo. Proc Natl Acad Sci USA. 1998;95:94829487. [PubMed] |
| 16. | Nestle FO, Alijagic S, Gilliet M, Sun Y, Grabbe S, Dummer R, Burg G, Schadendorf D. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat Med. 1998;4:328-332. [PubMed] |
| 17. | Holtl L, Zelle-Rieser C, Gander H, Papesh C, Ramoner R, Bartsch G, Rogatsch H, Barsoum AL, Coggin JH Jr, Thurnher M. Immunotherapy of metastatic renal cell carcinoma with tumor lysate-pulsed autologous dendritic cells. Clin Cancer Res. 2002;8:3369-3376. [PubMed] |
| 18. | Maier T, Tun-Kyi A, Tassis A, Jungius KP, Burg G, Dummer R, Nestle FO. Vaccination of patients with cutaneous T-cell lymphoma using intranodal injection of autologous tumor-lysate杙ulsed dendritic cells. Blood. 2003;102:2338-2344. [PubMed] |
| 19. | Mels AK, Mayen I, Egmond Mv M, Beelen RH, Meijer S, Richters CD. Reduction of liver metastases outgrowth by tumour antigen-pulsed dendritic cell vaccination. Comp Hepatol. 2004;3:S54. [PubMed] |
| 20. | Rains N, Cannan RJ, Chen W, Stubbs RS. Development of a dendritic cell(DC)- based vaccine for patients with advanced colorectal cancer. Hepatogastroenterology. 2001;48:347-351. [PubMed] |
| 21. | Klein C, Bueler H, Mulligan RC. Comparative analysis of genetically modified dendritic cells and tumor cells as therapeutic cancer vaccines. J Exp Med. 2000;191:16991708. [PubMed] |
| 22. | Wan Y, Bramson J, Pilon A, Zhu Q, Gauldie J. Genetically modified dentritic cells prime autoreactive T cells through a pathway independent of CD40L and interleukin 12: implications for cancer vaccines. Cancer Res. 2000;60:32473253. [PubMed] |
| 23. | Audibert F. Adjuvants for vaccines, a quest. Int Immunophar-macol. 2003;3:11871193. [PubMed] |
| 24. | Whiteside TL, Odoux C. Dendritia cell biology and cancer therapy. Cancer Immunol Immunother. 2004;53:240-248. [PubMed] |
| 25. | Srivastava P. Heat shock proteins and immune response: methods to madness. Methods. 2004;32:1-2. [PubMed] |
| 26. | Ueda G, Tamura Y, Hirai I, Kamiguchi K, Ichimiya S, Torigoe T, Hiratsuka H, Sunakawa H, Sato N. Tumor-derived heat shock protein 70-pulsed dendritic cells elicit tumor-specific cytotoxic T lymphocytes(CTLs)and tumor immunity. Cancer Sci. 2004;95:248253. [PubMed] |
| 27. | Udono H, Yamano T, Kawabata Y, Ueda M, Yui K. Generation of cytotoxic T lymphocytes by MHC class I ligands fused to heat shock cognate protein 70. Int Immuno. 2001;13:1233-1242. [PubMed] |
| 28. | Prasad SJ, Farrand KJ, Matthews SA, Chang JH, McHugh RS, Ronchese F. Dendritic cells loaded with stressed tumor cells elicit long-lasting protective tumor immunity in mice depleted of CD4+CD25+ regulatory T cells. J Immunol. 2005;174:90-98. [PubMed] |
| 29. | Singh-Jasuja H, Hilf N, Arnold-Schild D, Schild H. The role of heat shock proteins and their receptors in the activation of the immune system. Biol Chem. 2001;382:629-636. [PubMed] |
| 30. | Srivastava PK, Amato RJ. Heat shock proteins: the Swiss army Knife vaccines against cancers and infectious agents. Vaccine. 2001;19:25902597. [PubMed] |
| 31. | Zheng H, Dai J, Stoilova D, Li Z. Cell surface targeting of heat shock protein gp96 induces dendritic cell maturation and antitumor immunity. J Immunol. 2001;167:6731-6735. [PubMed] |
| 32. | Kuppner MC, Gastpar R, Gelwer S, Nossner E, Ochmann O, Scharner A, Issels RD. The role of heat shock protein (hsp70) in dendritic cell maturation: hsp70 induces the maturation of immature dendritic cells but reduces DC differentiation from monocyte precursors. Eur J Immunol. 2001;31:1602-1609. [PubMed] |