修回日期: 2004-11-22
接受日期: 2004-11-29
在线出版日期: 2005-02-15
目的: 探讨将人双突变的二氢叶酸还原酶基因(DHFR)和胞苷脱氨基酶基因(CD)同时导入小鼠骨髓细胞中, 观察小鼠对大剂量氨甲喋呤(MTX)和阿糖胞苷(Ara-C)的耐受性, 研究骨髓耐受联合化疗的可行性.
方法: 以反转录病毒为载体, 将人双突变的二氢叶酸还原酶基因(DHFR)和胞苷脱氨基酶基因(CD)通过共培养转染入小鼠骨髓干细胞, 观察共培养后的骨髓细胞及受体小鼠骨髓移植后经药物处理后的骨髓细胞耐MTX及Ara-C CFU-GM生成情况; 转基因小鼠骨髓细胞提取的DNA, 用PCR检测转基因小鼠骨髓细胞耐药基因的表达; 观察转基因小鼠经大剂量MTX和Ara-C化疗后血象、体质量及生存率的变化.
结果: 骨髓移植前共培养后供体的骨髓细胞和骨髓移植后受体含有耐药基因(SFG-F/S-CD)的骨髓细胞均有耐药克隆的形成(14%, 20%; χ2分别为42.55, 44.26; P<0.01), 并明显增加了对MTX和Ara-C的耐受; 与对照组比较含双耐药基因组动物经大剂量化疗后, 生存率明显提高(χ2 = 7.42, P<0.01), 血象逐渐恢复正常; 转基因小鼠骨髓细胞经PCR检测, 显示有F/S-CD基因条带; 耐药基因转染后小鼠骨髓对MTX和Ara-C 的耐受明显增加.
结论: 双耐药基因可以进入小鼠骨髓细胞并且获得共表达, 提高了造血细胞对MTX和Aar-C的耐药性.
引文著录: 路平, 金锋, 陈波, 姚凡, 王舒宝, 陈峻青, 徐惠绵, 赵实诚. DHFR-CD双耐药基因增强转基因小鼠对大剂量化疗毒性的抵御能力. 世界华人消化杂志 2005; 13(4): 464-467
Revised: November 22, 2004
Accepted: November 29, 2004
Published online: February 15, 2005
AIM: To explore the feasibility of transferring dihydrofolate reductase- (DHFR) gene and cytidine deaminase (CD) fusion gene into mouse bone marrow (BM) cells to induce resistance to high dose methotrexate (MTX) and cytosine arabinoside (Ara-C), and to improve the tolerance of myelosuppression following combination chemotherapy.
METHODS: Human double-mutant DHFR-CD fusion gene was transferred into mouse BM cells by retroviral vector Granulocyte-macrophage colony-forming unit (CFU-GM) assay was performed for retrovirally infected and drug treated mouse BM cells. DNA was extracted from mouse BM, and the expression of drug resistant genes was examined by PCR.
RESULTS: Drug resistant colonies were formed by donor mouse BM cells co-cultured with the retrovirus producing cells, as well as the BM cells from recipient mice transplanted with the fusion gene transfected BM cells (CFU-GM of donor mice was 14%, χ2 = 42.55, P<0.01; CFU-GM of recipient mice was 20%, χ2 = 44.26, P<0.01). The drug resistance to both MTX and Ara-C was also increased in the recipient mice. The survival rate of gene transferred mice was significantly higher compared with the control mice χ2 = 7.42, P<0.01. Expression of the DHFR-CD fusion gene in the transfected mice was confirmed by PCR.
CONCLUSION: Double drug resistant genes can be integrated and expressed in mouse bone marrow cells; furthermore, they can increase the drug resistance to MTX and Ara-C.
- Citation: Lu P, Jin F, Chen B, Yao F, Wang SB, Chen JQ, Xu HM, Zhao SC. Protection against toxicity of high dose chemotherapy in mice transfected with double-mutant dihydrofolate reductase-cytidine deaminase gene. Shijie Huaren Xiaohua Zazhi 2005; 13(4): 464-467
- URL: https://www.wjgnet.com/1009-3079/full/v13/i4/464.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v13.i4.464
骨髓抑制限制了化疗药物的应用, 使之难以达到治愈或抑制肿瘤生长的剂量. 如何提高骨髓等正常细胞对化疗药物的耐受性, 减少化疗的毒副作用, 成为肿瘤化疗研究的焦点[1]. 我们将双突变的人二氢叶酸还原酶基因(dihydrofolate reductase, DHFR)和胸苷脱氨基酶基因(cytidieaminase, CD)同时导入小鼠骨髓细胞中, 观察小鼠对大剂量氨甲喋呤(methotrexate, MTX)和阿糖胞苷(cytosine arabinoside, Ara-C)的耐受性, 结果如下.
含耐药基因的反转录病毒载体SFG-CD(含人突变的胞嘧啶脱氨酶mCD), SFG-F/S-NeoR(含双突变"Phe22-Ser31"的二氢叶酸还原酶dmDHFR和耐新霉素基因NeoR), SFG-F/S-CD(含双突变的二氢叶酸还原酶dmDHFR与胞嘧啶脱氨酶), 转染入病毒产生细胞(amphotropic packaging cell, GP-Am12, 简称Am12), 分别构成AM12-SFG-F/S-CD, 简称为SFG-F/S-CD(图1). 以上载体及细胞系由赵实诚教授提供. BALB/c♂小鼠8-10周龄, 行5-FU尾静脉注射(150 mg/kg), 该药可杀伤成熟粒细胞而不影响造血祖细胞, 注射4 d后取其股骨、胫骨的骨髓细胞(2-5)×107, 制成单细胞悬液. 计数骨髓有核细胞总数, 使骨髓有核细胞和转基因之病毒产生细胞按1:1的比例, 置于含200 mL/L小牛血清和10 g/L谷氨酰胺, 100 g/L WEHI条件培养液中, 分两组(SFG-F/S-CD, Am12)在37 ℃ 50 mL/L CO2条件下共培养48 h. 含耐药基因病毒产生细胞系在共培养前2 h照射1.5 Gy. 共培养后收集骨髓细胞, 制成细胞悬液(在PBS平衡液中作为骨髓移植及CFU-GM培养用). 受体小鼠在骨髓移植前均经8 Gy致死量照射, 以完全破坏其自身造血功能, 观察被输入的造血细胞在体内增生及血液的耐药情况. 小鼠根据体质量随机分为两组: A组转染SFG-F/S-CD; B组阴性对照组, 被输入的骨髓细胞仅与AM12共培养不含耐药基因. 每只小鼠根据其组别, 在致死量照射后12 h尾静脉注射2×106骨髓细胞. 骨髓移植后4 wk每只小鼠ip MTX(20 mg/kg)和Ara-C(30 mg/kg)连续4 d. 期中及注射后(25 d, 30 d, 35 d, 40 d及45 d)观察血象、体质量及生存率的变化.
为了解造血干细胞同含耐药基因的病毒产生细胞共培养后, 在小鼠体外体内的基因表达和耐药CFU-GM生成情况(检测共培养后的骨髓细胞行体外实验, 受体小鼠骨髓移植和药物处理45 d后取骨髓细胞行体内实验). 培养分为两组: A组加MTX及Ara-C, Ara-C与MTX的终浓度分别为20 nmol/L及500 nmol/L; B组为对照组, 不加药物. 每组共6 mL培养液含骨髓细胞3×106, 平分为3个平皿进行培养, 培养液为IMDM, 含10 g/L甲基纤维素, 100 mL/L胎牛血清, 150 g/L WEHI条件培养液(小鼠造血增生因子), 10 g/L碳酸氢钠, 10 g/L丙酮酸钠, 10 g/L必需与非必需氨基酸, 5 g/L多种维生素及10万单位/L青链霉素等, 胎牛血清在加入混合培养液前经用1 mg/L胸苷酸磷酶37 ℃ 1 h处理, 以减少细胞外磷酸腺苷水平以至减少集落本底的产生. 培养皿置含50 mL/L CO237 ℃培养箱7-10 d后计数集落, 进行结果分析. 取经基因转染及药物处理后小鼠骨髓细胞, 按饱合氯化钠法提取DNA.50 mL的PCR反应体系终浓度为0.125 mmol/L dNTPs. 两种引物0.5 mmol/L, 0.05 MU/L Taq DNA聚合酶, 模板DNA 150 ng.PCR 扩增条件是: 94 ℃变性10 min, 94 ℃变性1 min, 58 ℃退火1 min, 72 ℃延伸2 min, 共40循环之后72 ℃延伸10 min.PCR产物在0.8 g/L琼脂糖凝胶中进行电泳, 照像分析. 检测该试验耐药基因SFG-F/S-CD的引物序例两端设计为: 5'-ACTTTGAAAGTGACACGTTT-3'5'-GCCAAACTCTCTCATGACTTGC-3'扩增结果分别为498 bp.
统计学处理 生存分析应用Log Rank 检验(SPSS10.0 统计软件), 计数资料行χ2检验, 计量资料经t检验.
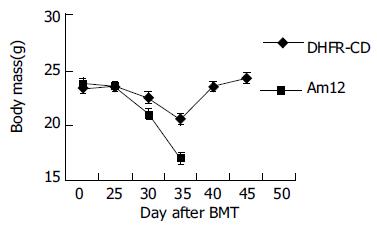
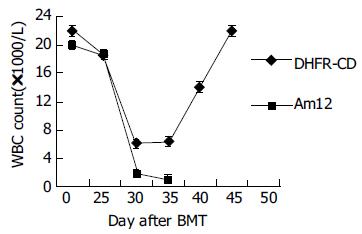
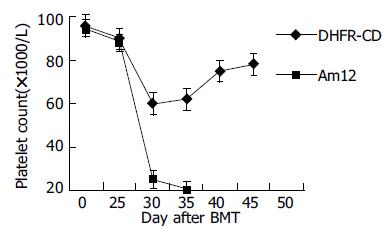
小鼠注射MTX和Ara-C后, 转染耐药基因的动物与对照相比, 动物存活率明显提高、体质量、白细胞和血小板恢复较快.
转染耐药基因后F/S-CD组小鼠在大剂量MTX和Ara-C处理后45 d存活率达67%(4/6), 对照组无存活, 经Log Rank检验(SPSS10.0统计软件)χ2=7.42, P<0.01. 小鼠注射MTX和Ara-C后, 体质量、白细胞和血小板均开始下降, F/S-CD组小鼠血象、体质量下降幅度较小而恢复较快, 45 d体质量、白细胞和血小板均恢复正常(图2-4).
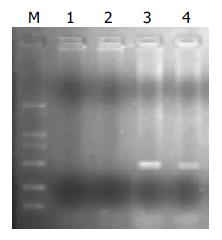
骨髓移植前供体的骨髓细胞和骨髓移植后受体的骨髓细胞含有耐药基因组均有耐药克隆的形成(表1). 骨髓移植前对照组注射终浓度为20 nmol/L和500 nmol/L 的MTX和Ara-C后, 对两种药物的耐药克隆分别为9%和0, 含双耐药基因F/S-CD组为56%和22%; 若两药同时加入两组耐药克隆分别为0和14%, 说明无耐药基因对两种药物的加入无耐药性, 而具有双耐药基因组则有一定程度的耐受性. 骨髓移植及化疗药物筛选后转染耐药基因组耐药克隆形成略有增加, 对照组分别为7%和0, F/S-CD组为60%和26%; 两药同时加入两组耐药克隆分别为0和20%.转基因小鼠骨髓细胞提取的DNA, 经PCR检测, 显示有F/S-CD基因条带, 说明动物耐药基因转染已得到分子生物学表达(图5).
如何提高骨髓造血细胞对化疗的耐受性, 一直是近年来人们所关注的焦点. 多药耐药基因(multiple drug resistance, MDR)是人们最早关注的肿瘤药物耐药基因[2]. 应用多药耐药基因(MDR1)转染保护骨髓造血细胞的基因治疗项目已进入临床试验阶段. 一些化疗药物耐药基因, 在体外及小鼠研究中证实对正常细胞具有化疗保护作用[3]. 理想的耐药基因应具备以下条件[4]: (1)提供多种药物耐药而非单一药物; (2)无免疫原性; (3)治疗基因片段较小; (4)该耐药基因耐特异药不宜毒性过大, 如一些学者认为MGMT和ALDH基因作用的烷化剂等化疗药物毒性过大[5]; (5)基因转染无突变及癌变产生. 近年来的实验研究证实, 二氢叶酸还原酶基因(dihydrofolate reductase, DHFR)[6-7] 及胞苷脱氨酶基因(cytidine deaminase, CD)[8]基本具备上述条件.
骨髓造血干细胞具有自我更新及定向分化的能力而成为基因治疗的理想靶细胞[9]. 耐药基因转导入正常造血干细胞是肿瘤基因治疗的一种策略. 逆转录病毒载体能实现在基因组内插入整合, 而成为造血干细胞基因导入的首选载体. 目前近70%的临床基因治疗试验均由逆转录病毒介导, 本实验用逆转录病毒转染小鼠骨髓细胞耐药克隆达20%.
双耐药基因导入造血干细胞为消除联合化疗导致的骨髓抑制提供了可能性, 较单耐药基因的转导更有意义. 我们用逆转录病毒载体将双耐药基因转导深入到动物实验水平, 进一步研究了dmDHFR-CD耐药基因转染后对小鼠造血功能的保护作用. 结果表明转基因小鼠骨髓对MTX和Ara-C的耐受明显增加, 实验组血象逐渐恢复, 而对照组造血则被抑制; 实验组小鼠体质量和生存期明显高于对照组. PCR分析, 转导后的小鼠骨髓细胞具有双耐药基因表达. 本研究证实逆病毒载体确能将两个外源耐药基因导入小鼠造血干细胞并同时进行表达产生药物抗性, 为进一步的耐药基因保护治疗动物实验打下了基础, 为临床超剂量化疗以求最大限度杀伤肿瘤细胞并能多次重复化疗提供了一个较好的动物模型, 可能为提高肿瘤的有关化疗疗效提供有用的参考资料. 但如何选择更合适或更多的耐药基因联合转导, 耐药基因导入造血干细胞后是否能否长期稳定表达, 以及对靶细胞的生理功能有何影响等问题均有待于进一步的研究.
编辑: 潘伯荣 审读:张海宁
| 1. | Takebe N, Zhao SC, Ural AU, Johnson MR, Banerjee D, Diasio RB, Bertino JR. Retroviral transduction of humandihydropyrimidine dehydrogenase cDNA confers resistance to 5-fluorouracil in murine hematopoietic progenitor cellsand human CD34+-enriched peripheral blood progenitor cells. Cancer Gene Ther. 2001;8:966-973. [PubMed] [DOI] |
| 2. | Sorrentino BP, Brandt SJ, Bodine D, Gottesman M, Pastan I, Cline A, Nienhuis AW. Selection of drug-resistant bonemarrow cells in vivo after retroviral transfer of human MDR1. Science. 1992;257:99103. [PubMed] [DOI] |
| 3. | Capiaux GM, Budak-Alpdogan T, Takebe N, Mayer-Kuckuk P, Banerjee D, Maley F, Bertino JR. Retroviral transductionof a mutant dihydrofolate reductase-thymidylate synthase fusion gene into murine marrow cells confers resistance toboth methotrexate and 5-fluorouracil. Hum Gene Ther. 2003;14:435-446. [PubMed] [DOI] |
| 4. | Eliopoulos N, Al-Khaldi A, Beausejour CM, Momparler RL, Momparler LF, Galipeau J. Human cytidine deaminase as anex vivo drug selectable marker in gene-modified primary bone marrow stromal cells. Gene Ther. 2002;9:452-462. [PubMed] [DOI] |
| 5. | Beausejour CM, Eliopoulos N, Momparler L, Le NL, Momparler RL. Selection of drug-resistant transduced cells withcytosine nucleoside analogs using the human cytidine deaminase gene. Cancer Gene Ther. 2001;8:669676. [PubMed] [DOI] |
| 6. | Asai S, Miyachi H, Kobayashi H, Takemura Y, Ando Y. Large diversity in transport-mediated methotrexate resistancein human leukemia cell line CCRF-CEM established in a high concentration of leucovorin. Cancer Sci. 2003;94:210-214. [PubMed] [DOI] |
| 7. | Banerjee D, Mayer-Kuckuk P, Capiaux G, Budak-Alpdogan T, Gorlick R, Bertino JR. Novel aspects of resistance todrugs targeted to dihydrofolate reductase and thymidylate synthase. Biochim Biophys Acta. 2002;1587:164-173. [PubMed] [DOI] |
| 8. | Sweeney CL, Frandsen JL, Verfaillie CM, McIvor RS. Trimetrexate inhibits progression of the murine 32Dp210 modelof chronic myeloid leukemia in animals expressing drug-resistant dihydrofolate reductase. Cancer Res. 2003;63:13041310. [PubMed] |