修回日期: 2004-10-02
接受日期: 2004-10-11
在线出版日期: 2005-02-01
目的: 观察血管生成素-2(angiopoietin-2)和金属基质蛋白酶-9(MMP-9)在胃癌组织中的表达, 并探讨其临床意义.
方法: 用免疫组化染色法检测了50例胃癌患者癌组织, 10例非癌胃黏膜组织中angiopoietin-2(Ang-2), MMP-9, CD-34蛋白的表达.
结果: 胃癌组织中Ang-2, MMP-9的阳性率分别为56%和60%, 显著高于非癌胃黏膜. 他们的表达水平与胃癌的分化程度呈负相关(25% vs 71%, χ2 = 9.18, P<0.01; 31% vs 74%, χ2 = 8.10, P<0.01), 与肿瘤的TNM分期(30% vs 78%, P<0.01; 35% vs81%, P<0.01), 淋巴结转移(39% vs 70%, P<0.05; 43% vs 74%, P<0.05), 微血管密度(Ang-2: 74.9±11.8 vs 65.6±11.5,P<0.05)成正相关(此外, MMP-9的表达水平还与肿瘤浸润的深度成正相关(33% vs 79%, χ2 = 10.73, P<0.01). Ang-2、MMP-9在胃癌中的阳性表达率之间存在一定相关性(χ2 = 3.0, P <0.01).
结论: Ang-2、MMP-9在胃癌的侵袭与转移中起重要作用, 并且他们之间存在相互诱导或是协同效应, 其共位表达可作为判断预后的指标.
引文著录: 钱颉, 刘南植, 程胜平. 胃癌组织Angiopoietin-2及MMP-9表达的意义. 世界华人消化杂志 2005; 13(3): 299-302
Revised: October 2, 2004
Accepted: October 11, 2004
Published online: February 1, 2005
AIM: To investigate the expression of angiopoietin-2 (Ang-2) and matrix metalloproteinase-9 (MMP-9) in gastric carcinoma.
METHODS: The expression of Ang-2 and MMP-9 was detected in tissues of gastric cancer (n = 50) and non-cancerous gastric mucosa (n = 10) by immunohistochemistry.
RESULTS: The positive rates of Ang-2 and MMP-9 in gastric cancer were 56% and 60%, which were significantly higher than those in non-cancerous mucosa. The level of Ang-2 and MMP-9 expression had a negative correlation with the differentiation degree (25% vs 71%, χ2 = 9.18, P<0.01; 31% vs 74%, χ2 = 8.10, P<0.01), but a positive correlation with TNM stage (30% vs 78%, P<0.01; 35% vs 81%, P<0.01), lymphatic metastasis (39% vs 70%, P<0.05; 43% vs 74%, P<0.05), or microvascular density (Ang-2: 74.911.8 vs 65.611.5, P<0.05). In addition, the level of MMP-9 expression was correlated with the invasion depth (33% vs 79%, P<0.01). Also, there was a significant correlation between the expression of Ang-2 and MMP-9 (χ2 = 13.0, P<0.01).
CONCLUSION: Ang-2 and MMP-9 play a synergistic role in the invasion and metastasis of gastric carcinoma. Their colocalization may be a useful indicator for the prognosis of the disease.
- Citation: Qian J, Liu NZ, Cheng SP. Expression of Angiopoietin-2 and Matrix metalloproteinase-9 in gastric carcinoma. Shijie Huaren Xiaohua Zazhi 2005; 13(3): 299-302
- URL: https://www.wjgnet.com/1009-3079/full/v13/i3/299.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v13.i3.299
Ang-2是新发现的一种与肿瘤血管生成相关的细胞因子, 他能特异性地与Tie-2结合, 促进新血管的生成[1-8]. 研究表明, 在肿瘤组织远处浸润的区域, Ang-2高表达的同时, 也伴随着MMPs的高表达[9-10]. 为此我们通过免疫组化技术对50例经手术治疗的胃癌患者组织标本进行血管生成素-2(Ang-2)、基质金属蛋白酶-9(MMP-9)和微血管密度(MVD)的检测, 探讨Ang-2, MMP-9与胃癌生物学特性的关系, 并对二者的相关性进行分析, 旨在为临床治疗及判断预后提供理论依据.
2003-01/2003-10同济医院根治性手术切除胃癌标本50例. 男30例, 女20例, 年龄28-84(平均年龄52.5)岁. 高/中分化组16例, 低分化组34例: 按UICC新TNM分期标准分为Ⅰ期9例, Ⅱ14例, Ⅲ/Ⅳ27例; 有淋巴结转移27例, 阴性23例. 另良性溃疡胃切除组织标本10例作为对照组. 标本为常规石蜡包埋切片, 留做HE及免疫组化染色. 兔抗angiopoietin-2多克隆抗体(R&D公司产品, 1:200 PBS稀释), 兔抗MMP-9多克隆抗体(Neomakers公司产品, 1:300 PBS稀释), 鼠抗CD34mAb(Zymed公司产品, 1:150 PBS稀释), 相应的SP试剂盒购于北京中山公司.
免疫组化用常规SP法, 最后以DAB显色, 用PBS代替一抗做阴性对照. 以细胞胞质或血管内皮出现棕黄色颗粒, 且其着色强度高于背景非特异性染色者判断为阳性. 对于Ang-2与MMP-9免疫组化染色的判定以每例切片中阳性细胞数与全部细胞数的比例计算: 无阳性细胞为(-); 阳性细胞数<25%为(+); 26-75%为(++); ≥76%为(+++). CD34阳性为血管内皮细胞胞质着色, 采用Weidner[11]微血管记数方法: 在200×视野下计数阳性血管数, 每例选择10个不重复视野计算单位视野(200×视野)的平均微血管数, 即间质微血管密度(MVD), 单位为个/200×, 结果用mean±SD表示. 根据数据类型分别使用χ2检验、t检验及相关分析, 检测标准a = 0.05, a = 0.01.
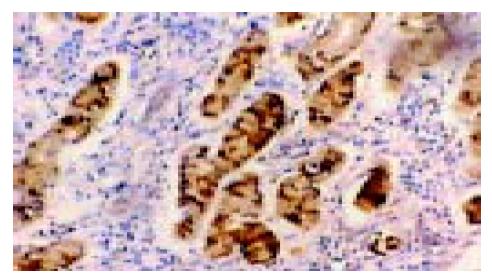
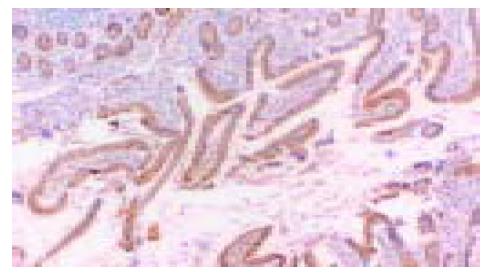
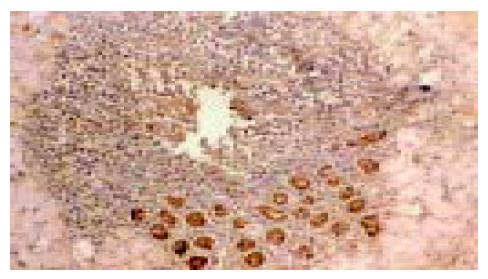
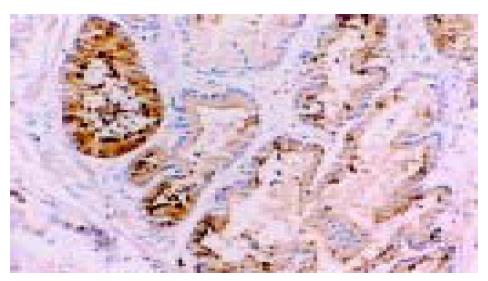
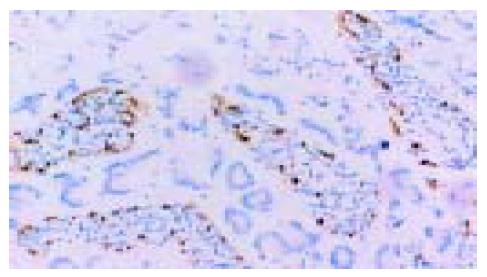
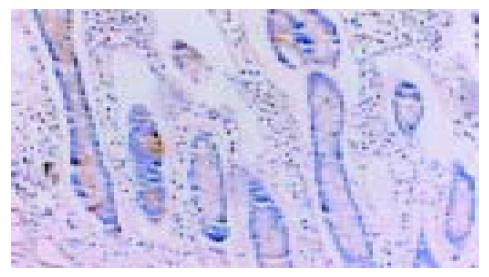
在50例胃癌组织中有28例Ang-2染色阳性, 30例MMP-9染色阳性. Ang-2、MMP-9阳性表达主要位于肿瘤细胞胞质, 呈散在、片状或灶状分布, 大部分都位于癌肿侵袭正常组织的边缘以及血管的附近, 也有侵犯到胃壁淋巴小结的肿瘤细胞呈阳性反应(图1-6). 另外, Ang-2在血管内皮细胞, 平滑肌细胞也有少量的表达, 但其阳性强度低于肿瘤细胞; MMP-9在纤维母细胞、巨噬细胞、血管内皮细胞也呈部分弱阳性表达. 在10例非癌胃黏膜组织中, 有9例Ang-2, MMP-9完全不表达仅1例呈弱阳性表达.
Ang-2的表达强度与胃癌的浸润深度无明显相关性(P>0.05), 与胃癌的分化程度、临床分期、淋巴结转移密切相关(25% vs 71%, χ2 = 9.18, P<0.01; 30% vs 78%, χ2 = 11.28, P<0.01; 39% vs 70%, χ2 = 4.92, P<0.05). MMP-9的表达强度则与胃癌的浸润深度、分化程度、临床分期以及淋巴结转移都有显著的相关性(33% vs79%, χ2 = 10.73, P<0.01; 31% vs 74%, χ2 = 8.10, P<0.01; 35% vs 81%, χ2 = 11.29, P<0.01; 43% vs 74%, χ2 = 4.84, P<0.05)(表1).
| 参数 | n | Ang-2 | MMP-9 | ||
| -/+ | +/+++ | -/+ | +/+++ | ||
| 分化程度高/中 | 16 | 12(7.0) | 4(9.0) | 11(6.4) | 5(9.6) |
| 低 | 34 | 15(15.0) | 24(19.0) | 9(13.6) | 25(20.4)b |
| TNM分期Ⅰ/Ⅱ期 | 23 | 16(10.1) | 7(12.9) | 15(9.2) | 8(13.8) |
| Ⅲ/Ⅵ期 | 27 | 6(11.9) | 21(15.1) | 5(10.8) | 22(16.2)b |
| 浸润深度T1/T2 | 21 | 12(9.2) | 9(11.8) | 14(8.4) | 7(12.6) |
| T3/T4 | 29 | 10(12.8) | 19(16.2) | 6(11.6) | 23(17.4)b |
| 淋巴结转移(-)性 | 23 | 14(10.1) | 9(12.9) | 13(9.2) | 10(13.8) |
| (+)性 | 27 | 8(11.9) | 19(15.1) | 7(10.8) | 20(16.2)a |
Ang-2阳性组与Ang-2阴性组比较, MVD值有显著性差异(74.9±11.8vs 65.6±11.5, t = 2.79, P<0.05). 在Ang-2阳性率高的切片, 微血管密度也高, 而且, 在恶性程度越高的病例, Ang-2的表达越高, 间质中的血管也越丰富. 说明Ang-2的表达可以诱导胃癌组织新血管的生成, 且与肿瘤的恶性程度有一定相关性.
在28例Ang-2阳性病例中, 有23例MMP-9阳性, 在22例Ang-2阴性病例中有15例MMP-9阴性, 即Ang-2(+)/MMP-9(+)23例, Ang-2(+)/MMP-9(-)5例, Ang-2(-)/MMP-9(-)15例, Ang-2(-)/MMP-9(+)7例. 组间比较, 具有显著性差异(χ2 = 13.0, P<0.01).
新生血管的形成在肿瘤发生发展过程中有重要的作用[12-15], Angiopoietin-2是新发现的一种促进肿瘤血管生成的细胞因子. 他特异性表达于人体多种肿瘤边缘的血管重建区, 参与肿瘤血管新生的起始及延续过程, 从而影响肿瘤的生长与转移[16-20]. 金属基质蛋白酶(MMPs)则是一类发现比较早的, 结构中含有Zn2+、Ca2+等金属离子的蛋白水解酶类, 能水解胶原蛋白、弹性蛋白、氨基葡聚糖等多种细胞外基质, 参与多种肿瘤的侵袭与转移[21-29]. MMP-9是基质金属蛋白酶家族的很重要的一员, 他与MMP-2同属于明胶酶, 其作用底物是Ⅳ、Ⅴ型胶原、凝胶、弹力蛋白等. 有研究表明Ang-2, MMP-9分别与胃癌的发生、发展有一定的相关性, 但是目前, 对于Ang-2, MMP-9在胃癌的血管形成、侵袭与转移过程中所起的作用是否具有协同性, 尚无研究. 我们用免疫组化的方法检测了50例胃癌患者癌组织及5例非癌胃黏膜组织中Ang-2、MMP-9蛋白的表达. 结果表明, 在10例非癌胃黏膜中仅有1例有少量细胞表达Ang-2及MMP-9, 阳性表达率低于25%, 且该病例为胃窦部巨大溃疡行胃大部切除手术, 尚不能排除其有恶变倾向. 在50例胃癌中有28例Ang-2强阳性表达, 其中9例早期胃癌有4例阳性表达, 与晚期相比具有统计学意义(P<0.05), 表明Ang-2能出现在胃癌早期. Ang-2的表达主要位于癌灶边缘与正常组织交界处以及血管的附近, 多数表达于肿瘤细胞胞质, 也有少量的单核细胞和血管内皮细胞着色, 说明不仅是内皮细胞, 胃癌细胞也可以分泌Ang-2. 我们的研究结果还表明, Ang-2的表达还与胃癌的分化程度、病理分级、MVD及淋巴结转移密切相关. 由此可见, Ang-2在胃癌的发生、发展及远处转移过程中起着重要的作用. 这与Eoth et al[30]的研究结果一致. 在50例胃癌中有30例MMP-9强阳性表达, 其表达与胃癌的分化程度、病理分级、浸润深度及淋巴结转移也有一定相关性. 由于肿瘤细胞存在着异质性, 所以Ang-2、MMP-9在肿瘤组织的表达并不均一.
我们的实验结果还表明Ang-2及MMP-9在胃癌组织中的表达有一定的相关性, 在28例Ang-2阳性病例中, 有23例MMP-9阳性. Ang-2表达阳性率高的切片, MMP-9的阳性率也高, 并且表达的部位基本一致. 但究竟是不是同Hu[9]的研究一样, Ang-2的表达可以上调MMP-9的表达, 还需要进一步的研究. 倘若这种猜想是正确的, 则可为胃癌的基因治疗开辟新的道路. 由于Ang-2除女性生殖器官外, 在机体的正常组织不表达或低表达, 若作为抗肿瘤治疗的靶点, 预计全身影响也比较小. 另一方面, Ang-2, MMP-9在胃癌的侵袭和转移中起着重要的作用, 其共位表达可望成为判断预后的指标.
编辑: 潘伯荣 审读:张海宁
| 1. | Asahara T, Chen DH, Takahashi T, Fujikawa K, Kearney M, Magner M, Yancopoulos GD, Isner JM. Tie2 receptorligands, angiopoietin-1 and angiopoietin-2, modulate VEGF-Induced postnatal neovascularization. Circulation Research. 1998;83:233-240. [PubMed] [DOI] |
| 2. | Ferrara N. Vascular endothelial growth factor: molecular and biological aspects. Curr Top Micobiol Immunol. 1999;237:1-30. [PubMed] [DOI] |
| 3. | Huang XL, Takakura N, Suda T. In vitro effects of angiopoietins and VEGF on hematopoietic and endothelial cells. Biochem Biophys Res Commun. 1999;264:133-138. [PubMed] [DOI] |
| 4. | Mezquita J, Mezquita P, Montserrat P, Mezquita B, Francone V, Vilagrasa X, Mezquita C. Genomic structure andalternative splicing of chicken angiopoietin-2. Biochem Biophys Res Commun. 2000;275:643-651. [PubMed] [DOI] |
| 5. | Horner A, Bord S, Kelsall AW, Coleman N, Compston JE. Tie2 ligands angiopoietin-1 and angiopoietin-2 arecoexpressed with vascular endothelial cell growth factor in growing human bone. Bone. 2001;28:65-71. [PubMed] [DOI] |
| 6. | Kampfer H, Pfeilschifter J, Frank S. Expressional regulation of angiopoietin-1 and -2 and the tie-1 and -2 receptortyrosine kinases during cutaneous wound healing: a comparative study of normal and impaired repair. Lab Invest. 2001;81:361-373. [PubMed] [DOI] |
| 7. | Sato T, El-Assal ON, Ono T, Yamanoi A, Dhar DK, Nagasue N. Sinusoidal endothelial cell proliferation and expressionof angiopoietin/Tie family in regenerating rat liver. J Hepatol. 2001;34:690-698. [PubMed] [DOI] |
| 8. | Tscheudschilsuren G, Aust G, Nieber K, Schilling N, Spanel-Borowski K. Microvascular endothelial cells differ in basaland hypoxia-regulated expression of angiogenic factors and their receptors. Microvasc Res. 2002;63:243-251. [PubMed] [DOI] |
| 9. | Hu B, Guo P, Fang Q, Tao HQ, Wang D, Nagane M, Huang HJ, Gunji Y, Nishikawa R, Alitalo K. Angiopoietin-2 induces human glioma invasion through the activation of matrix metalloprotease-2. Proc Natl Acad Sci USA. 2003;100:8904-8909. [PubMed] [DOI] |
| 10. | Das A, Fanslow W, Cerretti D, Warren E, Talarico N, McGuire P. Angiopoietin/Tek interactions regulate mmp-9expression and retinal neovascularization. Lab Invest. 2003;83:1637-1645. [PubMed] [DOI] |
| 11. | Weidner N, Folkman J, Pozza F, Bevilacqua P, Allred EN, Moore DH, Meli S, Gasparini G. Tumor angiogenesis:a new significant and independent prognostic indicator in early-stage breast carcinoma. J Natl Cancer Inst. 1992;84:1875-1887. [PubMed] [DOI] |
| 13. | Gasparini G. Prognostic value of vascular endothelial growth factor in breast cancer. Oncologist. 2000;5:37-44. [PubMed] |
| 16. | Chen L, Yang Z, Wang G, Wang C. Expression of angiopoietin-2 gene and its receptor Tie2 in hepatocellular carcinoma. J Tongji Med Univ. 2001;21:228-230. [PubMed] [DOI] |
| 17. | Currie MJ, Gunningham SP, Turner K, Han C, Scott PA, Robinson BA, Chong W, Harris AL, Fox SB. Expression ofthe angiopoietins and their receptor Tie2 in human renal clear cell carcinomas; regulation by the von Hippel-Lindaugene and hypoxia. J Pathol. 2002;198:502-510. [PubMed] [DOI] |
| 18. | Gale NW, Thurston G, Davis S, Wiegand SJ, Holash J, Rudge JS, Yancopoulos GD. Complementary and coordinatedroles of the VEGFs and angiopoietins during normal and pathologic vascular formation. Cold Spring Harb SympQuant Biol. 2002;67:267-273. [PubMed] [DOI] |
| 19. | Xing L, Zhang Z, Xu Y. Expression of angiopoietin-2 gene in non-small cell lung cancer. J Huazhong Univ SciTechnolog Med Sci. 2003;23:362-364. [PubMed] [DOI] |
| 20. | Caine GJ, Blann AD, Stonelake PS, Stonelake PS, Ryan P, Lip GY. Plasma angiopoietin-1, angiopoietin-2 and Tie-2in breast and prostate cancer: a comparison with VEGF and Flt-1. Eur J Clin Invest. 2003;33:883-890. [PubMed] [DOI] |
| 23. | Kabashima A, Yao T, Sugimachi K, Tsuneyoshi M. Relationship between biologic behavior and phenotypic expressionin intramucosal gastric carcinomas. Hum Pathol. 2002;33:80-86. [PubMed] [DOI] |
| 24. | Wroblewski LE, Pritchard DM, Carter S, Varro A. Gastrin-stimulated gastric epithelial cell invasion: the role andmechanism of increased matrix metalloproteinase 9 expression. Biochem J. 2002;365:873-879. [PubMed] [DOI] |
| 25. | Caudroy S, Polette M, Nawrocki-Raby B, Cao J, Toole BP, Zucker S, Birembaut P. EMMPRIN-mediated MMP regulationin tumor and endothelial cells. Clin Exp Metastasis. 2002;19:697-702. [PubMed] [DOI] |
| 26. | Shin EY, Ma EK, Kim CK, Kwak SJ, Kim EG. Src/ERK but not phospholipase D is involved in keratinocyte growthfactor-stimulated secretion of matrix metalloprotease-9 and urokinase-type plasminogen activator in SNU-16human stomach cancer cell. J Cancer Res Clin Oncol. 2002;128:596-602. [PubMed] [DOI] |
| 27. | Nyberg P, Moilanen M, Paju A, Sarin A, Stenman UH, Sorsa T, Salo T. MMP-9 activation by tumor trypsin-2 enhancesin vivo invasion of human tongue carcinoma cells. J Dent Res. 2002;81:831-835. [PubMed] [DOI] |
| 28. | Albo D, Shinohara T, Tuszynski GP. Up-regulation of matrix metalloproteinase 9 by thrombospondin 1 in gastric cancer. J Surg Res. 2002;108:51-60. [PubMed] [DOI] |
| 30. | Etoh T, Inoue H, Tanaka S, Barnard GF, Kitano S, Mori M. Angiopoietin-2 is related to tumor angiogenesis ingastric carcinoma: possible in vivo regulation via induction of proteases. Cancer Res. 2001;61:2145-2153. [PubMed] |