修回日期: 2005-11-01
接受日期: 2005-11-12
在线出版日期: 2005-12-15
目的: 探讨应激对大鼠结肠神经系统nNOS表达的影响.
方法: SD大鼠30只随机分为对照组, 应激组和L-NAME组, 采用水浸-束缚应激(WRS)动物模型, 用免疫组织化学ABC法检测nNOS在大鼠结肠黏膜下神经丛和肌间神经丛的表达, 应用计算机图像分析系统对其表达进行定量分析.
结果: 与对照组比较, 应激组黏膜下神经丛和肌间神经丛的nNOS阳性神经元的灰度值明显减少(P = 0.02或P = 0.005), 阳性神经元细胞数的平均密度增加(P = 0.04或P = 0.01), 表达增强, 且在黏膜上皮细胞、固有层淋巴细胞也有nNOS表达. L-NAME组黏膜下神经丛和肌间神经丛的nNOS阳性神经元的灰度值较应激组增加(P = 0.04), 平均密度下降(P = 0.04或P = 0.03), 表达减弱, 而与对照组比较均无明显差异(P >0.05).
结论: 应激可引起大鼠结肠神经系统nNOS表达增强, 提示一氧化氮(NO)在应激所致的结肠功能失调中可能起重要作用.
引文著录: 李玉梅, 陆国明. 应激对大鼠结肠神经系统 nNOS 表达的影响. 世界华人消化杂志 2005; 13(23): 2766-2769
Revised: November 1, 2005
Accepted: November 12, 2005
Published online: December 15, 2005
AIM: To investigate the effect of stress on the expression of neuronal nitric oxide synthase (nNOS) in colonic nerv-ous system in rats.
METHODS: Thirty male SD rats were randomly divided into control group, stress group and NG-nitro-L-arginine methyl ester (L-NAME) group. The rat model of water immersion-restraint stress (WRS) was established. The expression of nNOS in colonic submucous plexus and myenteric plexus in the rats was examined by immuno-histochemical staining and analyzed by computer image analysis system.
RESULTS: nNOS immune-positive substance was mostly expressed in the neurons of submucous plexus and myenteric plexus. In comparison with that in contr-ol group, the gray value of nNOS positive neurons in submucous plexus and myenteric plexus was signifi-cantly decreased (P = 0.02 or P = 0.005), and the den-sity of nNOS positive neurons was increased markedly (P = 0.04 or P = 0.01) in stress group. Moreover, nNOS expression in mucosal epithelial cells and lamina pro-pria lymphocytes were also observed. In comparison with that in stress group, the gray value of nNOS posi-tive neurons in submucous plexus and myenteric plexus was increased (P = 0.04), and the density of nNOS positive neurons was decreased (P = 0.04 or P = 0.03) in L-NAME group. nNOS expression was not significantly different between the rats of L-NAME and control group (P >0.05).
CONCLUSION: WRS can increase the expression of nNOS in colonic nervous system in rats, which suggests nitric oxide (NO) may play an important role in WRS-induced function disorder of colon.
- Citation: Li YM, Lu GM. Effect of stress on expression of neuronal nitric oxide synthase in colonic nervous system in rats. Shijie Huaren Xiaohua Zazhi 2005; 13(23): 2766-2769
- URL: https://www.wjgnet.com/1009-3079/full/v13/i23/2766.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v13.i23.2766
肠道存在复杂的肠神经系统(enteric nervous system, ENS). ENS接受中枢神经系统的调控, 并通过各种神经介质调节肠道平滑肌的运动和腺体分泌, 维持其正常生理功能. 近年来发现应激参与肠道某些功能性疾病、自身免疫性疾病(如肠易激综合征, IBS)的发生、发展[1-3]. 一氧化氮(nitric oxide, NO)是一种新型气体信号分子和神经递质, 可舒张胃肠平滑肌, 调节肠道血液循环, 参与细胞信号传递, 在肠道的生理及病理过程中具有重要的生物学功能[4-6]. 有关应激对nNOS在大鼠肠神经系统表达的影响尚未见相关报道. 我们采用水浸-束缚应激(water immersion-restraint stress, WRS)动物模型[7], 应用免疫组织化学方法, 旨在探讨应激对大鼠结肠神经系统nNOS表达的影响, 并为IBS动物模型的制作提供新的思路.
左旋硝基精氨酸甲酯(NG-nitro-L-arginine methyl ester, L-NAME)购自美国Sigma公司, 使用前用生理盐水稀释(浓度为5 g/L); 兔抗鼠nNOS多克隆抗体(工作浓度为1∶100)和免疫组织化学试剂盒(即用型SABC)均购自武汉博士德生物工程有限公司. 清洁级♂SD大鼠30只(浙江省实验动物中心提供), 体质量200-220 g, 随机分成对照组、应激组和L-NAME组, 每组10只. 实验前大鼠禁食24 h, 禁水1 h. L-NAME组大鼠在应激前30 min ip L-NAME 20 mg/kg, 对照组和应激组大鼠均于实验前30 min ip等体积生理盐水. 然后将应激组和L-NAME组大鼠束缚于鼠板上并垂直浸入20±1℃水浴中, 水面平大鼠胸骨剑突水平, 持续4 h. 对照组不施加应激.
大鼠用1%(m/v)戊巴比妥钠(30 mg/kg)颈后皮下注射麻醉. 剖腹后在距回肠末端3 cm处剪取长度为1.0 cm的结肠标本. 切开肠管后用生理盐水漂洗, 放入4%(m/v)多聚甲醛溶液固定, 常规石蜡包埋后制作4 µm切片, 用于HE染色和免疫组织化学分析. 免疫组织化学采用SABC法, 主要步骤包括: 石蜡切片脱蜡、梯度酒精脱水; 30 mL/L H2O2甲醇液室温孵育20 min灭活内源性过氧化物酶; 枸橼酸缓冲液(0.01 mol/L, pH 6.0)中92-100℃抗原修复20 min, 冷却; 30 mL/L正常山羊血清37℃孵育20 min; 一抗工作液(兔抗鼠nNOS)冰箱内4℃孵育24 h; 生物素标记的羊抗鼠IgG 37℃孵育20 min; 链霉卵白素37℃孵育20 min; SABC室温孵育20 min; DAB显色, 黄色或棕黄色产物为阳性标记; 苏木素复染; 常规脱水、透明、封片; 光镜下观察. 以0.01 mol/L PBS(pH 7.4)代替一抗作为阴性对照. 每张切片随机连续选取不重叠的5个高倍视野(×400), 计数每个高倍视野内黏膜下神经丛和肌间神经丛的阳性神经元数, 取其平均值分别代表两种神经丛内阳性神经元的平均密度(阳性神经元细胞数/高倍视野); 并用Nikon Act-1图像分析仪和Image-Pro Plus 4.5图像分析软件测量阳性神经元的灰度值, 取平均值代表阳性神经元的灰度值以反映nNOS蛋白的相对含量.
统计学处理 所有数据以均数±标准差(mean±SD)表示, 运用SPSS 10.0统计软件进行t检验, P<0.05为有统计学意义.
光镜下对照组、应激组和L-NAME组大鼠结肠组织结构完整, 均未见明显损伤性改变.
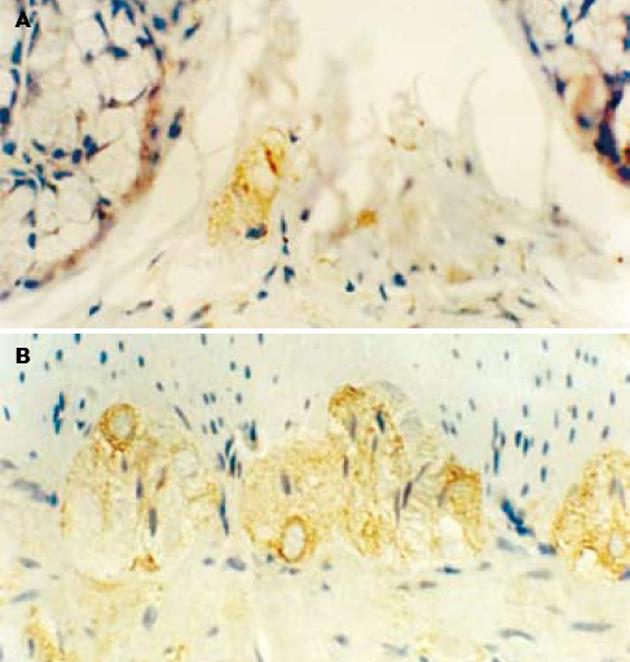
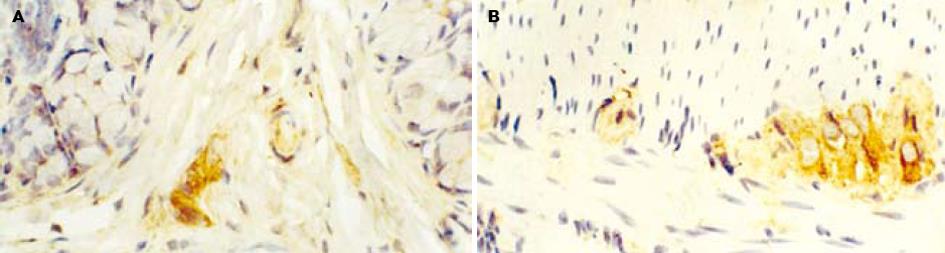
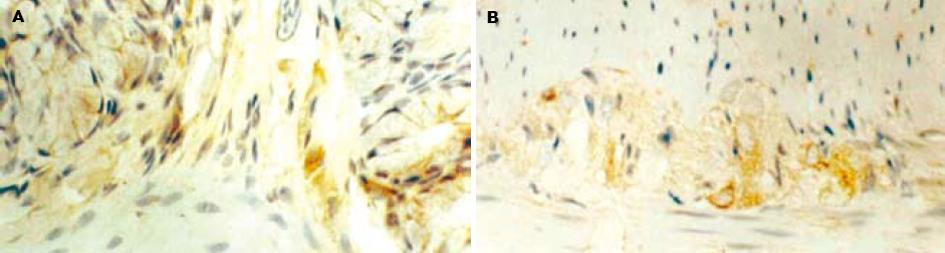
对照组大鼠nNOS免疫阳性产物主要见于结肠黏膜下神经丛和肌间神经丛的神经元, 定位于细胞质(图1). 应激组nNOS免疫阳性产物除在上述结构表达外, 黏膜上皮细胞、固有层淋巴细胞也有表达(图2). L-NAME组nNOS免疫阳性产物主要见于结肠黏膜下神经丛和肌间神经丛的神经元(图3).
结肠黏膜下神经丛nNOS阳性神经元灰度值和平均密度的变化: 应激组nNOS阳性神经元灰度值较对照组明显减少(P<0.05), 阳性神经元的平均密度高于对照组(P<0.05), 即nNOS表达增强. 与应激组相比, L-NAME组黏膜下神经丛的nNOS阳性神经元灰度值增加(P<0.05), 平均密度降低(P<0.05), 即nNOS表达减弱. 结肠肌间神经丛nNOS阳性神经元灰度值和平均密度的变化: 应激组肌间神经丛的nNOS阳性神经元灰度值较对照组显著减少(P<0.01), 平均密度高于对照组(P<0.05), 即nNOS表达明显增强. 与应激组相比, L-NAME组肌间神经丛的nNOS阳性神经元灰度值增加(P<0.05), 平均密度降低(P<0.05), 即nNOS表达明显减弱(表1).
NO是近年来发现的一种小分子气体信号分子和重要的神经递质. 内源性NO以L-精氨酸(L-Arg)为底物, 在NADPH、O2和原卟啉Ⅸ等因子辅助下, 由一氧化氮合酶(NOS)作用下产生. NO具有高度脂溶性, 合成后以扩散方式到达靶细胞, 与细胞中可溶性鸟甘酸环化酶(SGC)结合, 通过改变SGC的空间构型提高酶活性, 使细胞内环磷酸鸟苷(cGMP)生成增多, 激活依赖cGMP蛋白激酶的钙泵, 从而参与细胞间信息传递[8,9]. NOS是NO合成过程中唯一的限速酶, 其活性依赖于NADPH、Ca2+和钙调蛋白, 可被左旋精氨酸结构类似物(如L-NAME)竞争性抑制[10,11]. NOS包括三种亚型: 神经型NOS(neuronal NOS, nNOS)、内皮型NOS(endothelial NOS, eNOS)和诱导型NOS(inducible NOS, iNOS). 前两者合称为结构型NOS(constitutive NOS, cNOS). 酶细胞化学方法研究发现[12-14], NOS广泛分布于哺乳动物的肠道组织, 但此方法不能区分cNOS与iNOS. 我们采用免疫组化方法显示, nNOS主要分布于大鼠结肠黏膜下神经丛和肌间神经丛神经元, 定位于细胞质. 上述结果为揭示nNOS在大鼠肠道中的分布规律提供了形态学资料, 并进一步证实NO是结肠重要的信号分子和神经递质.
结肠的神经支配较为复杂, 其运动形式也比小肠复杂且多变[15,16]. 结肠肌间神经丛包括初级、二级及三级神经丛. 初级神经丛与二级神经丛相连形成网络, 其神经纤维穿入环行肌, 并直接支配环行肌的运动, 而纵行肌则由三级肌间丛支配. 肌间神经丛部分神经纤维伸至肠黏膜后形成感觉末梢. 而黏膜下神经丛的神经元不仅支配肠黏膜和黏膜肌层, 有些神经元还伸至肌间神经丛. 因此, 我们推测, 在生理状态下, 黏膜下神经丛内NO主要与结肠黏膜肌的收缩、腺体分泌和黏膜感觉有关, 而肌间神经丛NO主要参与了对结肠环行肌运动的调节.
研究表明, 应激通过神经体液调节, 常导致胃肠功能失调, 表现为胃肠运动、分泌和消化转运时间的改变[17-19]. 然而, 应激引起或加重结肠功能紊乱的发病机制尚不清楚. 有关NO与胃肠动力学的研究显示, 胃肠组织均可在cNOS催化下产生NO, 参与对肠道平滑肌收缩的非胆碱能非肾上腺素能(nonadrenergic noncholinergic, NANC)神经调节: NO含量减少时, 胃肠运动加快; 反之, 胃肠运动减慢[20]. 本研究显示, 水浸-束缚应激后黏膜下神经丛和肌间神经丛内nNOS阳性神经元的表达增强, 而预先用NOS抑制剂L-NAME处理可逆转nNOS的表达结果. 我们推测, 水浸-束缚应激状态下, 大鼠结肠黏膜下神经丛和肌间神经丛的神经元nNOS表达增强, 由nNOS催化L-Arg生成的NO含量增加, 扩散至周围靶细胞后, 不仅抑制平滑肌的收缩, 导致结肠运动减弱, 而且抑制了结肠黏膜肌的收缩、腺体分泌和黏膜感觉.
不同的应激往往产生不同的胃肠运动效应[21-25]. 激怒、恐惧、痛性刺激和高强度训练可使健康人的胃排空延迟, 结肠运动增强; 动物经束缚制动、电刺激和强迫游泳等急性应激后, 也可产生胃肠排空延迟. 近年来研究发现应激可诱发、加重IBS的症状[26-28], 导致结肠动力增强(腹泻型IBS)或减弱(便秘型IBS), 但至今尚无一种成熟的IBS动物模型, 因此有关IBS发病机制的基础研究较少. 本研究结果显示WRS后肠神经系统nNOS表达增强, 提示应激状态下抑制性神经递质NO产生增加, 参与了对肠道平滑肌活动的抑制, 但未引起结肠组织学变化. 因此, 我们认为WRS可作为研究IBS肠道动力异常的较为理想的动物模型.
电编: 张敏 编辑: 菅鑫妍 审读: 张海宁
| 1. | Verleye M, Gillardin JM. Effects of etifoxine on stress-induced hyperthermia, freezing behavior and colonic motor activation in rats. Physiol Behav. 2004;82:891-897. [PubMed] [DOI] |
| 2. | Locke GR 3rd, Weaver AL, Melton LJ 3rd, Talley NJ. Psycho-social factors are linked to functional gastrointestinal disorders: a population based nested case-control study. Am J Gastroenterol. 2004;99:350-357. [PubMed] [DOI] |
| 3. | Murray CD, Flynn J, Ratcliffe L, Jacyna MR, Kamm MA, Emm-anuel AV. Effect of acute physical and psychological stress on gut autonomic innervation in irritable bowel syndrome. Gastroenterology. 2004;127:1695-1703. [PubMed] [DOI] |
| 5. | Fritz E, Hammer J, Schmidt B, Eherer AJ, Hammer HF. Stimul-ation of the nitric oxide-guanosine 3', 5'-cyclic monophosphate pathway by sildenafil: effect on rectal muscle tone, distensib-ility, and perception in health and in irritable bowel syndrome. Am J Gastroenterol. 2003;98:2253-2260. [PubMed] |
| 6. | Shah V, Lyford G, Gores G, Farrugia G. Nitric oxide in gastroi-ntestinal health and disease. Gastroenterology. 2004;126:903-913. [PubMed] [DOI] |
| 7. | Nishioka T, Iyota K, Nakayama T, Suemaru S, Numata Y, Has-himoto K. Effects of ether-laparotomy and water immersion-restraint stress on CRH concentration in the hypothalamus, ex-trahypothalamic tissues and peripheral blood. Endocr J. 1993;40:213-220. [PubMed] [DOI] |
| 8. | Lowenstein CJ, Dinerman JL, Snyder SH. Nitric oxide: a phy-siologic messenger. Ann Intern Med. 1994;120:227-237. [PubMed] [DOI] |
| 9. | Bredt DS. Endogenous nitric oxide synthesis: biological functi-ons and pathophysiology. Free Radic Res. 1999;31:577-596. [PubMed] [DOI] |
| 10. | Aiko S, Fuseler J, Grisham MB. Effects of nitric oxide synthase inhibition or sulfasalazine on the spontaneous colitis observed in HLA-B27 transgenic rats. J Pharmacol Exp Ther. 1998;284:722-727. [PubMed] |
| 11. | Vardareli E, Dundar E, Angin K, Saricam T, Inal M. Effects of intrarectal and intraperitoneal N(G)-nitro-L-arginine methyl ester treatment in 2,4,6-trinitrobenzenesulfonic acid induced colitis in rats. Exp Toxicol Pathol. 2003;55:271-276. [PubMed] [DOI] |
| 12. | Nichols K, Staines W, Krantis A. Nitric oxide synthase distribution in the rat intestine: a histochemical analysis. Gastroenterology. 1993;105:1651-1661. [PubMed] [DOI] |
| 13. | Wilhelm M, Batori Z, Pasztor I, Gabriel R. NADPH-diaphorase positive myenteric neurons in the ileum of guinea-pig, rat, rabbit and cat: a comparative study. Eur J Morphol. 1998;36:143-152. [PubMed] [DOI] |
| 14. | Bagyanszki M, Roman V, Fekete E. Quantitative distribution of NADPH-diaphorase-positive myenteric neurons in different segments of the developing chicken small intestine and colon. Histochem J. 2000;32:679-684. [PubMed] [DOI] |
| 15. | Schemann M. Control of gastrointestinal motility by the "gut brain"-the enteric nervous system. J Pediatr Gastroenterol Nutr. 2005;41:S4-6. [PubMed] [DOI] |
| 16. | Grundy D, Schemann M. Enteric nervous system. Curr Opin Gastroenterol. 2005;21:176-182. [PubMed] [DOI] |
| 17. | Monnikes H, Tebbe JJ, Hildebrandt M, Arck P, Osmanoglou E, Rose M, Klapp B, Wiedenmann B, Heymann-Monnikes I. Role of stress in functional gastrointestinal disorders. Evidence for stress-induced alterations in gastrointestinal motility and sensitivity. Dig Dis. 2001;19:201-211. [PubMed] [DOI] |
| 18. | Tache Y, Perdue MH. Role of peripheral CRF signalling path-ways in stress-related alterations of gut motility and mucosal function. Neurogastroenterol Motil. 2004;16:137-142. [PubMed] [DOI] |
| 19. | Bhatia V, Tandon RK. Stress and the gastrointestinal tract. J Gastroenterol Hepatol. 2005;20:332-339. [PubMed] [DOI] |
| 20. | De Winter BY, Boeckxstaens GE, De Man JG, Moreels TG, Herman AG, Pelckmans PA. Effect of adrenergic and nitrergic blockade on experimental ileus in rats. Br J Pharmacol. 1997;120:464-468. [PubMed] [DOI] |
| 21. | Cao SG, Wu WC, Han Z, Wang MY. Effects of psychological stress on small intestinal motility and expression of cholecy- stokinin and vasoactive intestinal polypeptide in plasma and small intestine in mice. World J Gastroenterol. 2005;11:737-740. [PubMed] [DOI] |
| 22. | Nakade Y, Tsuchida D, Fukuda H, Iwa M, Pappas TN, Takaha-shi T. Restraint stress delays solid gastric emptying via a central CRF and peripheral sympathetic neuron in rats. Am J Physiol Regul Integr Comp Physiol. 2005;288:R427-432. [PubMed] [DOI] |
| 23. | Verleye M, Gillardin JM. Effects of etifoxine on stress-induced hyperthermia, freezing behavior and colonic motor activation in rats. Physiol Behav. 2004;82:891-897. [PubMed] [DOI] |
| 24. | Barreau F, Cartier C, Ferrier L, Fioramonti J, Bueno L. Nerve growth factor mediates alterations of colonic sensitivity and mucosal barrier induced by neonatal stress in rats. Gastroenterology. 2004;127:524-534. [PubMed] [DOI] |
| 25. | Tabosa A, Yamamura Y, Forno ER, Mello LE. A comparative study of the effects of electroacupuncture and moxibustion in the gastrointestinal motility of the rat. Dig Dis Sci. 2004;49:602-610. [PubMed] [DOI] |
| 26. | Quigley EM. Disturbances of motility and visceral hypersensi-tivity in irritable bowel syndrome: biological markers or epiphenomenon. Gastroenterol Clin North Am. 2005;34:221-233. [PubMed] [DOI] |
| 27. | Delvaux M. Alterations of sensori-motor functions of the diges-tive tract in the pathophysiology of irritable bowel syndrome. Best Pract Res Clin Gastroenterol. 2004;18:747-771. [PubMed] [DOI] |
| 28. | Ehlert U, Nater UM, Bohmelt A. High and low unstimulated salivary cortisol levels correspond to different symptoms of functional gastrointestinal disorders. J Psychosom Res. 2005;59:7-10. [PubMed] [DOI] |