修回日期: 2004-10-18
接受日期: 2004-10-27
在线出版日期: 2005-01-15
目的: 探讨胰岛素样生长因子-1对大鼠肝纤维化形成的影响.
方法: 50只SD大鼠随机编入4组, 正常组和橄榄油组各10只, 模型组15只, IGF-1干预组15只. 40%CCL4橄榄油溶液制备大鼠肝纤维化模型, 4 wk后, 以IGF-1 2 μg/kg干预, 共2 wk. 观察大鼠存活率、体重变化及纤维化形成率; 光镜下观察大鼠肝组织病理及胶原纤维变化; 采用Western-blot法检测肝组织中α-肌动蛋白(α-SMA)含量.
结果: IGF-1干预组大鼠存活率高于模型组, 体重(301.5±32.4 g)明显高于模型组(252.1±24.1 g, P<0.05), 肝纤维化形成率较模型组低, 光镜下肝组织纤维化程度明显改善, 胶原含量明显下降. IGF-1干预组中肝组织α-SMA含量明显低于模型组.
结论: IGF-1具有抗大鼠肝纤维化作用, 这可能与其改善肝功能、抑制HSC活化等有关.
引文著录: 万荣, 吴云林, 乔敏敏, 章永平, 付爱芬, 辛美珍, 孔雷, 张奕, 刘炳亚. 胰岛素样生长因子-1 对大鼠肝纤维化形成的影响. 世界华人消化杂志 2005; 13(2): 211-213
Revised: October 18, 2004
Accepted: October 27, 2004
Published online: January 15, 2005
AIM: To investigate the effect of insulin-like growth factor-I (ICF-I) on hepatic fibrosis in rats and its possible mechanism.
METHODS: Fifty Sprague-Dawley rats were randomly divided into 4 groups: normal group (n = 10), olive oil group (n = 10), model group (n = 15) and ICF-I group (n = 15). The hepatic fibrosis model was induced by 40% CCl4. The rats with hepatic fibrosis were injected with IGF-1 subcutaneously four weeks later (2 μg/kg, every other day for 2 wk). The survival rate, fibrotic degree and body weight of the rats were inspected. The histopathological changes of collagenous fiber were examined under optical microscope. The content of α-smooth muscle actin (α-SMA) in the liver tissue were detected by Western-blotting.
RESULTS: In comparison with those in model group, the weights of the rats in ICF-I group were significantly higher (252.1±24.1 g vs 301.5±32.4 g, P < 0.05); the survival rate was higher and the fibrotic degrees were remarkably lower; the content of collagen and α-SMA in the liver tissue were significantly reduced.
CONCLUSION: IGF-1 can protect rats against hepatic fibrosis, which may be related to its role in improving hepatic function and inhibiting HSC activation.
- Citation: Wan R, Wu YL, Qiao MM, Zhang YP, Fu AF, Xin MZ, Kong L, Zhang Y, Liu BY. Effect of insulin-like growth factor-I on hepatic fibrosis in rats. Shijie Huaren Xiaohua Zazhi 2005; 13(2): 211-213
- URL: https://www.wjgnet.com/1009-3079/full/v13/i2/211.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v13.i2.211
IGF-I是由70个氨基酸残基组成的单链多肽, 对肝纤维化发生、发展起着十分重要的作用[1]. 我们探讨胰岛素样生长因子-1抗大鼠肝纤维化的作用及其机制, 为临床应用提供理论依据.
纯系、♂SD大鼠50只, 体质量200±20 g, 由中国科学院上海实验动物中心提供; 橄榄油和CCl4(上海生化试剂厂), IGF-1(Sigma公司), 小鼠抗大鼠α-SMA单抗(武汉博士德公司); 7FR-180A电泳槽, Mini Protean 3电转移仪, PowerPac 300稳压稳流电泳仪, Fluors Mutilmager图像分析仪(Bio-Rad公司).
SD大鼠50只随机编入4组. 正常组和橄榄油组各10只, 分别于SC生理盐水和橄榄油3 mL/kg, 隔日一次, 首次加倍, 共6 wk; 模型组15只, 400 mL/L CCl4的橄榄油溶液, 按3 mL/kg皮下注射, 隔日1次, 首次加倍, 共6 wk; IGF-1干预组15只, 制模方法与模型组相同, 4 wk后加用IGF-1 20 μg/kg皮下注射, 共2 wk. HE染色观察大鼠肝病理学改变, 比较肝纤维化程度; 胶原纤维染色法观察肝胶原含量. 采用Western-blot法检测肝组织中α-SMA含量.
统计学处理 计量资料以mean±SD表示, 采用方差分析判断组间差异; 所有统计均经SAS6.12统计软件处理完成, P<0.05为有统计学意义.
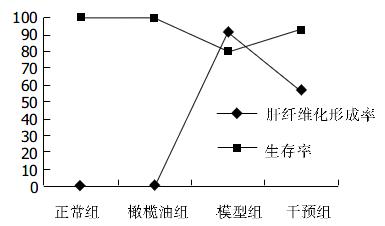
IGF-1干预组大鼠体质量(301.5g±32.4 g)明显高于模型组(252.1g±24.1 g, P<0.05). IGF-1干预组大鼠存活率高于模型组, 肝纤维化形成率较模型组低(图1)
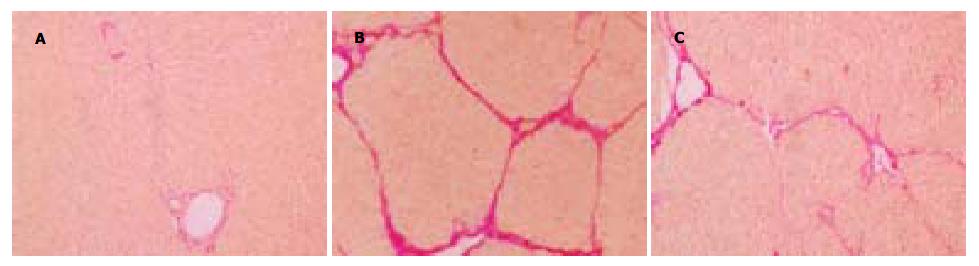
模型组HE染色可见多数正常肝小叶结构破坏或消失, 形成完整假小叶, 纤维隔内有大量单核、淋巴、嗜酸性细胞等浸润. IGF-1干预组肝细胞变性坏死明显减轻, 纤维间隔变细, 数量明显减少, 假小叶形成不完全. 正常组和橄榄油组仅见汇管区血管胶原染色. 模型组胶原染色可见红色胶原纤维大量增生, 汇管区-中央静脉区纤维间隔宽大, 增生的肝细胞被胶原纤维包绕、分割成大小不等的假小叶. IGF-1干预组有不同程度改善, 仅见少量胶原纤维间隔形成, 假小叶不完全形成(图2).
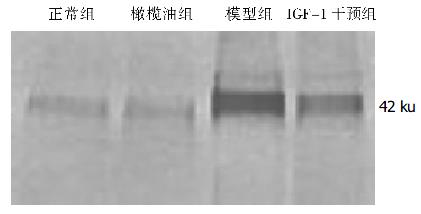
正常组和橄榄油组肝组织中α-SMA含量少, 且两组间差异不明显; 模型组肝组织中α-SMA含量显著高于正常对照组和橄榄油组, IGF-1干预组肝组织中α-SMA含量高于正常组和橄榄油组. IGF-1干预组肝组织中α-SMA含量明显低于模型组(图3).
IGF-I是由70个氨基酸残基组成的单链多肽, 主要在肝脏合成. 他是机体广泛存在的细胞有丝分裂和分化成熟的促进剂, 在细胞的生长、分化及代谢的调节中有着重要的作用, 参与了胚胎发育、机体生长、创伤愈合及肿瘤发生等过程[2-5]. 研究表明, IGF-1是肝星状细胞等成纤维样细胞的丝裂因子, 对肝纤维化发生、发展起着十分重要的作用, 与肝纤维化时肝功能状态、营养状况及其预后等密切相关[6]. Castilla-Cortazar et al[7-8]在四氯化碳诱导的大鼠肝纤维化模型验证IGF-I对肝脏的保护作用时发现, IGF-I能明显提高肝纤维化大鼠血浆白蛋白水平, 改善肝功能, 减轻肝纤维化程度. 本研究发现, IGF-1干预组大鼠肝脏病理学改变程度较模型组明显减轻, 表现为肝脏假小叶形成不完全, 胶原形成少, 纤维化程度减低, 提示IGF-1具有体内抗纤维化的作用.
在肝损伤及各种慢性肝病时, 肝星状细胞(HSC)被激活, 细胞内维生素A脂滴减少或消失, 其表型由静息型转变为激活型, 即HSC转换为肌成纤维细胞[9].α-SMA是肌动蛋白的一种, 在肌成纤维细胞中含量丰富, 当HSC转化为肌成纤维细胞时, 肝脏内α-SMA含量增加, 因此α-SMA的变化被看成HSC活化与否的标志, HSC的激活、增生是肝纤维化重要环节[10-11]. HSC活化对肝纤维化形成和门脉压力变化有着十分重要的作用[12]. 体外研究表明IGF-1有促进HSC活化、增生并分泌细胞外基质的作用[13-14]. 我们发现, IGF-1干预组大鼠肝内α-SMA产生较模型组明显减少, 表明体内IGF-1具有抑制HSC活化的作用, 这似与体外试验相矛盾. 究其原因, 体外试验单纯考虑的是IGF-1对HSC一对一的作用, 作用简单, 而体内环境复杂, 肝纤维化的发生和发展过程受多种因子和多种细胞的影响, 是一种复杂的网络系统, IGF-1可能通过减轻肝内炎性反应, 降低肝硬化鼠脂质过氧化物, 减轻肝内氧化损伤, 减少HSC活化的刺激因素等[15], 达到抑制HSC活化的目的.
关于IGF-1与肝纤维化的相关报道仅见于少数的动物实验[16]. 目前临床IGF-I仅用于糖尿病及肾脏病患者的治疗, 且研究仍是初步的, 并发现具有潜在的危险性. 本研究也是建立在动物实验的基础上, 进一步明了IGF-I与肝纤维化的关系, 为IGF-I用于临床抗肝纤维化及降门脉压治疗提供了新的理论基础. 我们相信, IGF-1有良好的临床治疗肝硬化患者的应用前景, 但尚需要一定过程.
编辑: 潘伯荣 审读: 张海宁
| 1. | Sanz S, Pucilowska JB, Liu S, Rodríguez-Ortigosa CM, Lund PK, Brenner DA, Fuller CR, Simmons JG, Pardo A, Martínez-Chantar ML. Expression of insulin-like growth factor I by activated hepatic stellate cells reduces fibrogenesis and enhances regeneration after liver injury. Gut. 2005;54:134-141. [PubMed] |
| 2. | Scavo LM, Karas M, Murray M, Leroith D. Insulin-like growth factor-I stimulates both cell growth and lipogenesis during differentiation of human mesenchymal stem cells into adipocytes. J Clin Endocrinol Metab. 2004;89:3543-3553. [PubMed] |
| 3. | Johnsen SP, Sørensen HT, Thomsen JL, Grønbaek H, Flyvbjerg A, Engberg M, Lauritzen T. Markers of fetal growth and serum levels of insulin-like growth factor (IGF) I, -II and IGF binding protein 3 in adults. Eur J Epidemiol. 2004;19:41-47. [PubMed] |
| 4. | Mattera D, Capuano G, Colao A, Pivonello R, Manguso F, Puzziello A, D'Agostino L. Increased IGF-I : IGFBP-3 ratio in patients with hepatocellular carcinoma. Clin Endocrinol (Oxf). 2003;59:699-706. [PubMed] |
| 5. | Mazziotti G, Sorvillo F, Morisco F, Carbone A, Rotondi M, Stornaiuolo G, Precone DF, Cioffi M, Gaeta GB, Caporaso N. Serum insulin-like growth factor I evaluation as a useful tool for predicting the risk of developing hepatocellular carcinoma in patients with hepatitis C virus-related cirrhosis: a prospective study. Cancer. 2002;95:2539-2545. [PubMed] |
| 6. | Vyzantiadis T, Theodoridou S, Giouleme O, Harsoulis P, Evgenidis N, Vyzantiadis A. Serum concentrations of insulin-like growth factor-I (IGF-I) in patients with liver cirrhosis. Hepatogastroenterology. 2003;50:814-816. [PubMed] |
| 7. | Castilla-Cortázar I, Pascual M, Urdaneta E, Pardo J, Puche JE, Vivas B, Díaz-Casares A, García M, Díaz-Sánchez M, Varela-Nieto I. Jejunal microvilli atrophy and reduced nutrient transport in rats with advanced liver cirrhosis: improvement by Insulin-like Growth Factor I. BMC Gastroenterol. 2004;4:12. [PubMed] |
| 8. | Castilla-Cortazar I, Garcia M, Muguerza B, Quiroga J, Perez R, Santidrian S, Prieto J. Hepatoprotective effects of insulin-like growth factor I in rats with carbon tetrachloride-induced cirrhosis. Gastroenterology. 1997;113:1682-1691. [PubMed] |
| 9. | Buniatian GH. Stages of activation of hepatic stellate cells: effects of ellagic acid, an inhibiter of liver fibrosis, on their differentiation in culture. Cell Prolif. 2003;36:307-319. [PubMed] |
| 10. | Shibata N, Watanabe T, Okitsu T, Sakaguchi M, Takesue M, Kunieda T, Omoto K, Yamamoto S, Tanaka N, Kobayashi N. Establishment of an immortalized human hepatic stellate cell line to develop antifibrotic therapies. Cell Transplant. 2003;12:499-507. [PubMed] |
| 11. | Kinnman N, Francoz C, Barbu V, Wendum D, Rey C, Hultcrantz R, Poupon R, Housset C. The myofibroblastic conversion of peribiliary fibrogenic cells distinct from hepatic stellate cells is stimulated by platelet-derived growth factor during liver fibrogenesis. Lab Invest. 2003;83:163-173. [PubMed] |
| 12. | Zhang RP, Zhang WH, Xue DB, Wei YW. Morphology of portal hypertension at the early stage of liver damage induced by CCl4: an experimental study with dogs. Zhonghua Yixue Zazhi. 2004;84:1118-1121. [PubMed] |
| 13. | Saile B, DiRocco P, Dudas J, El-Armouche H, Sebb H, Eisenbach C, Neubauer K, Ramadori G. IGF-I induces DNA synthesis and apoptosis in rat liver hepatic stellate cells (HSC) but DNA synthesis and proliferation in rat liver myofibroblasts (rMF). Lab Invest. 2004;84:1037-1049. [PubMed] |
| 14. | Scharf JG, Dombrowski F, Novosyadlyy R, Eisenbach C, Demori I, Kübler B, Braulke T. Insulin-like growth factor (IGF)-binding protein-1 is highly induced during acute carbon tetrachloride liver injury and potentiates the IGF-I-stimulated activation of rat hepatic stellate cells. Endocrinology. 2004;145:3463-3472. [PubMed] |
| 15. | García-Fernández M, Castilla-Cortázar I, Díaz-Sánchez M, Díez Caballero F, Castilla A, Díaz Casares A, Varela-Nieto I, González-Barón S. Effect of IGF-I on total serum antioxidant status in cirrhotic rats. J Physiol Biochem. 2003;59:145-146. [PubMed] |
| 16. | Cantürk NZ, Cantürk Z, Ozden M, Dalçik H, Yardimoglu M, Tülübas F. Protective effect of IGF-1 on experimental liver cirrhosis-induced common bile duct ligation. Hepatogastroenterology. 2003;50:2061-2066. [PubMed] |