修回日期: 2005-09-01
接受日期: 2005-09-06
在线出版日期: 2005-10-15
目的: 研究瘦素及SCD-1在高脂饮食引起非酒精性脂肪肝形成及其药物治疗中的作用.
方法: 42只SD大鼠分成正常组、高脂组和干预组(自高脂饮食喂养8 wk起用罗格列酮进行干预16 wk). 酶联免疫法测定血清瘦素, RT-PCR实时荧光分析大鼠肝SCD-1 mRNA与β-actin mRNA的比值.
结果: 肝组织HE染色显示高脂组大鼠肝脏内有弥漫性肝细胞脂肪变性, 8 wk达到脂肪肝诊断标准. 8、24 wk高脂组大鼠血清瘦素水平升高, 与正常组相比有显著性差异(8 wk: 5.29±1.83 mg/L vs 3.06±1.35 mg/L,P<0.05; 24 wk: 7.89±3.01 mg/L vs 3.09±1.52 mg/L, P<0.05); 肝SCD-1 mRNA与β-actin mRNA的比值明显下降(8 wk: 0.37±0.25 vs 0.82±0.34, P<0.05). 干预组与高脂组相比, 血清瘦素水平下降(5.95±3.31 mg/L vs 7.89±3.01 mg/L, P>0.05); 肝SCD-1 mRNA表达明显增强(SCD-1/β-actin: 1.02±0.11 vs 0.52±0.22, P<0.01).
结论: 长期高脂饮食可导致血清瘦素水平升高, 其通过下调肝SCD-1表达促进非酒精性脂肪肝的形成. 罗格列酮可降低血清瘦素水平, 上调肝SCD-1的表达, 减轻因高脂饮食引起的非酒精性肝脂肪变.
引文著录: 陆元善, 范建高, 方继伟, 丁晓东, 杨兆瑞. 瘦素及硬脂酰 CoA 去饱和酶-1在高脂饮食大鼠非酒精性脂肪肝发病中的作用. 世界华人消化杂志 2005; 13(19): 2327-2331
Revised: September 1, 2005
Accepted: September 6, 2005
Published online: October 15, 2005
AIM: To explore the roles of leptin and stearoyl-CoA desaturase-1 (SCD-1) in the formation of nonalcoholic fatty liver disease (NAFLD) induced by a fat-rich diet, and their responses after medication.
METHODS: Forty-two SD rats were divided into control, experimental, and therapeutic group. The rats in the experimental and therapeutic group were fed with a fat-rich diet to establish NAFLD model, and the rats in the therapeutic group were treated with rosiglitazone for 16 wk from the 9th week of the fat-rich diet. The concentration of serum leptin was measured by enzyme linked immunosorbent assay (ELISA). The ratio of liver SCD-1 mRNA to β-actin mRNA was analyzed by real-time fluorescence reverse transcription polymerase chain reaction (RT-PCR).
RESULTS: Diffusive hepatic steatosis was observed under light microscope in the rats fed by the fat-rich diet at 8 wk. The serum leptin level of rats was notably higher in the experimental than that in control group at 8 and 24 wk ( 8 wk: 5.29±1.83 μg/L vs 3.06±1.35 μg/L, P < 0.05; 24 wk: 7.89±3.01 μg/L vs 3.09±1.52 μg/L, P < 0.05), and the ratio of SCD-1 mRNA/β-actin mRNA was significantly decreased (8 wk: 0.37±0.25 vs 0.82±0.34, P < 0.05). The concentration of serum leptin in the rosiglitazone treated rats was decreased as compared with that in the experimental rats (5.95±3.31 μg/L vs 7.89±3.01 μg/L, P>0.05), and the expression of SCD-1 mRNA in liver were increased (SCD-1/β-actin: 1.02±0.11 vs 0.52±0.22, P < 0.01).
CONCLUSION: A fat-rich diet can lead to the increase of serum leptin, which may promote the formation of NAFLD by down-regulation of SCD-1. Rosiglitazone can decrease the level of serum leptin, so as to protect the liver against NAFLD to some extent.
- Citation: Lu YS, Fan JG, Fang JW, Ding XD, Yang ZR. Roles of leptin and stearoyl-CoA desaturase-1 in pathogenesis of nonalcoholic fatty liver disease induced by a fat-rich diet. Shijie Huaren Xiaohua Zazhi 2005; 13(19): 2327-2331
- URL: https://www.wjgnet.com/1009-3079/full/v13/i19/2327.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v13.i19.2327
非酒精性脂肪肝主要是由于中性脂肪在肝脏中沉积所引起, 而高脂饮食是非酒精性脂肪肝形成的原因之一[1]. 瘦素是由脂肪细胞分泌的抑制进食、刺激能量消耗、维持体重防止脂毒性对非脂肪组织损伤的激素[2-4]. 硬脂酰CoA去饱和酶(stearoyl-CoA desaturase, SCD, E.C. 1.14.99.5)是单不饱和脂肪酸(monounsaturated fatty acid, MUFA)生物合成的限速酶, 是瘦素作用目的基因之一, 在脂肪酸代谢中起中心调节作用[3,5-8]. 以往的研究均采用突变小鼠或大鼠探讨瘦素或SCD对脂肪肝形成的影响[9-13], 我们通过对高脂饮食引起肝脏脂肪病变及非酒精性脂肪肝形成后治疗SD大鼠动物模型的研究, 以阐明瘦素和SCD在脂肪肝形成、发展及治疗中作用, 从而为非酒精性脂肪肝的预防和治疗提供理论指导.
1.1.1 动物: ♀SD大鼠, 购自于中科院上海实验动物中心斯莱克公司, 体重150 g左右(140-160 g).
1.1.2 试剂: 胆固醇纯品: 上海生化试剂商店. 猪油: 自备. Taq DNA聚合酶、dNTP及逆转录试剂均为Promega产品. 大鼠瘦素酶联免疫测定试剂: 美国DSL公司.
1.1.3 仪器: MJ扩增仪、低温离心机和全自动酶标仪.
1.2.1 非酒精性脂肪肝模型: 42只大鼠正常喂养1 wk后, 随机分成2组, 对照组18只, 高脂组24只. 对照组以普通饲料喂养, 高脂组以2%胆固醇、10%猪油和88%标准大鼠饲料构成的高脂饲料喂养. 于实验开始后第4、8和24 wk分别处死6只对照组和6只高脂组大鼠. 大鼠以0.1 g/kg体重氯胺酮予以麻醉, 腹主动脉采血. 称取肝湿重后, 迅速从肝右叶固定部位切取1块肝组织, 以40 g/L的中性福尔马林固定后制备成石蜡切片, 剩余肝组织经标记后投入液氮罐中冷冻保存备用. 治疗组为高脂喂养8 wk起, 在饮水中加入2.7 mg/kg/d的罗格列酮干预.
1.2.2 总RNA提取: 从液氮中取出肝组织, 称取0.1 g, 放入Eppendorf管中, 加入1 mL Trizol, 研磨匀浆. 匀浆中加入0.2 mL氯仿, 剧烈震荡15 s, 室温孵育5 min. 4 ℃, 12000 r/min离心15 min, 将无色水相移至另一Eppendorf管. 加入0.5 mL异丙醇, 室温孵育15 min, 再4 ℃ 12000 r/min离心10 min, 弃上清. 沉淀加入750 mL/L乙醇1 mL, 洗涤沉淀物. 4 ℃ 7500 r/min离心5 min, 弃上清. 沉淀用20 mL DEPC水溶解. 取1 mL溶解好的RNA溶液,DEPC水稀释至100 mL, 微量分光光度计检测RNA纯度和浓度.
1.2.3 RT-PCR
1.2.3.1 RT: 按产品说明书操作. 取总RNA 2 mg, 加入随机引物2 mL, 用无RNA酶去离子水添至总体积12 mL, 70 ℃,5 min. 取出立即置冰中, 快速冷却. 然后加入10 mmol/L dNTP 0.8 mL、10X缓冲液2 mL、25 mmol/L MgCl2 3 mL、RNA酶抑制剂0.7 mL和逆转录酶0.5 mL, 37 ℃,20 min, 42 ℃,30 min, 最后95 ℃,3 min, 4 ℃,5 min. 产物cDNA -80 ℃保存备用.
1.2.3.2 Real-time PCR: 取10X缓冲液2.5 mL、25 mmol/L MgCl2 3 mL、10 mmol/L dNTP 0.8 mL、cDNA 2 mL、25 mmol/L 引物各0.5 mL、Taq DNA聚合酶1 u, 20X SYBR green I 1.25 mL, 去离子水加至25 mL. 按95 ℃, 3 min变性, 然后95 ℃, 20 s、56 ℃, 30 s和72 ℃, 30 s, 40个循环. β-actin的引物为: 5'-AACCCTAAGGCCAACCGTGAAAAG-3'和5'-TCATGAGTAGTCTGTCAGGT-3'; SCD-1的引物为: 5'-TGCTGATGTGCTTCATCCTG-3'和5'-GGGAAACCAGGATATTCTCC-3'.
1.2.4 瘦素测定: 按试剂盒规定的操作规程在全自动酶标仪上完成测试, 测定波长450 nm.
统计学处理 4 wk及8 wk的对照组与高脂组对应指标用t检验, 24 wk的对照组、高脂组及罗格列酮干预组对应指标用ANOVA分析, 统计软件为SSPS 9.0.
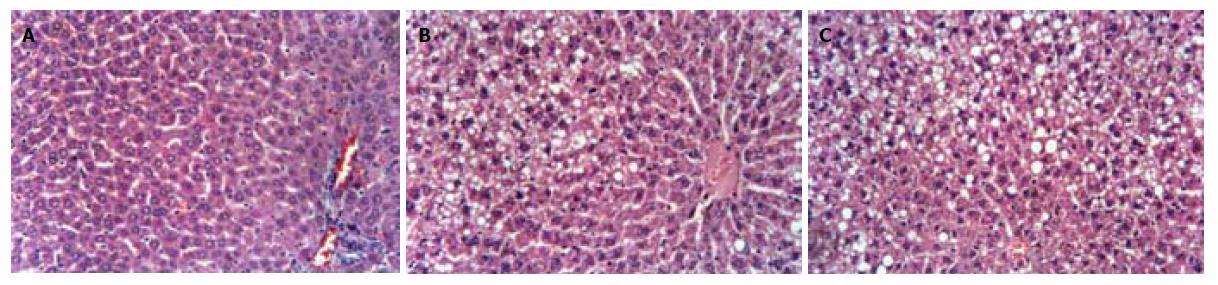
实验过程中未发生大鼠死亡, 2组大鼠体重均呈进行性增长. 4 wk高脂组大鼠的肝脏湿重与对照组有显著差异. 8 wk高脂组大鼠的体重、肝脏湿重和腹腔内脂肪均显著高于对照组(表1). 肝脏大体观察, 对照组大鼠肝脏形态、质地、颜色均正常; 高脂组大鼠的肝脏体积增大, 外形饱满圆钝, 色泽灰黄, 切面油腻, 质地较脆. 光镜下, 肝组织HE染色显示高脂组大鼠肝脏内有弥漫性肝细胞脂肪变, 8 wk达到脂肪肝诊断标准(图1). 24 wk高脂组呈中重度脂肪肝, 肝脏炎症程度较重[14].
SCD-1和β-actin的扩增产物分别为201 bp和241 bp. 两组大鼠血清瘦素水平及肝脏SCD-1mRNA水平在4 wk虽有差异, 但无统计学意义; 8 wk高脂组大鼠血清瘦素水平显著高于对照组, 肝脏SCD-1mRNA水平明显低于对照组(表1).
瘦素是由脂肪细胞分泌的抑制进食、刺激能量消耗、维持质量防止脂毒性对非脂肪组织损伤的激素[2-4,15], 正常情况下, 脂肪堆积引起瘦素分泌增多, 可诱导胰岛β细胞超极化, 抑制胰岛素分泌, 减少脂肪合成及储存[12,16,17]. Cohen et al研究显示SCD-1基因是瘦素信号的靶基因[18], SCD-1通过无氧氧化催化硬脂酰和软脂酰CoA形成油酰CoA和棕榈油酰CoA, 油酸是肝合成甘油三酯和胆固醇酯的必需脂肪酸, 甘油三酯和胆固醇酯是肝内装配和分泌VLDL的必需成份, 控制VLDL形成[3,7,18-20], SCD是调节VLDL水平的控制点. SCD主要有四种同功酶: SCD-1、SCD-2、SCD-3和SCD-4[19-21]. SCD-1主要存在于肝、肾、肺、心脏和脾. 因而, 研究瘦素及SCD-1与高脂饮食引起的脂肪肝及其治疗的关系, 对了解脂肪肝的形成机理及临床治疗均有帮助. 我们发现高脂饮食喂养可成功地塑造非酒精性脂肪肝模型. 大鼠腹腔内脂肪增多, 肝脏明显增大, 肝细胞内有脂肪颗粒沉积, 肝细胞的超微结构受到不同程度的损坏[22]. 大鼠质量增加, 同时血清瘦素水平升高, 与其它学者的研究结果相一致[23,24]. 瘦素增高可能与高脂喂养引起腹腔内脂肪增多或肝细胞发生了成脂性改变有关. 同时发现经一段时间喂养高脂饮食大鼠肝SCD-1 mRNA水平下降, SCD-1mRNA水平下降可能与高脂喂养引起的内源性高瘦素血症有关, Cohen et al[18]最近研究显示SCD-1基因是瘦素信号的靶基因, 瘦素抑制SCD-1基因的表达. 瘦素通过转录因子SREBP对SCD-1表达进行调节[17]. SCD是一种膜蛋白, 位于内质网上. 内质网的调节环路确保了与脂质平衡有关酶在转录和转录后水平的调节[19]. 腺病毒-导入引起高瘦素血症的正常鼠可引起脂肪组织和非脂肪组织中脂肪的减少和SREBP减少[23]. SREBP-1c是SREBP-1在肝中表达的主要异构体, 并调节成脂基因的表达, 更偏重于增强肝内包括SCD1在内的有关脂肪酸合成必需基因的转录[25,26]. SREBP-1c下降使肝细胞内SCD-1和脂肪组织的SCD-2转录降低[10], 血浆游离脂肪酸增加, 肝内多余的脂肪不能重新酯化并装配成VLDL排出, 脂质在肝内沉积; 血浆游离脂肪酸增加, 使肝细胞易受具有脂毒性的游离脂肪酸的损伤[12,16,17], 加速了脂肪肝的形成[27]. 瘦素对SCD-1的影响也可能通过神经肽(neuropeptide Y, NPY)起作用, NPY诱导肝乙酰CoA羧化酶和其它成脂酶基因的表达. 瘦素水平增高抑制下丘脑NPY基因表达, 使SCD-1表达下降[28,29]. 第4周高脂组血清瘦素水平较对照组低, 而肝SCD-1mRNA水平高于对照组, 但均无显著性差异, 可能与检测误差有关, 或由于高脂饮食引起血浆脂质水平升高, 肝SCD-1的表达代偿性升高.
研究发现罗格列酮治疗后, 大鼠质量、脂肪含量及血清瘦素水平均有所下降, 同时SCD-1mRNA与β-actin mRNA比值上升. 这可能与罗格列酮的药理作用有关. 作为胰岛素敏感增强剂, 可增加细胞对胰岛素的敏感性, 调节细胞内SERBP的表达[30,31], 使SCD-1表达增加, 肝内多余的饱和脂肪酸转变成不饱和脂肪酸, 经VLDL排出. SCD-1催化活性与其表达呈正比, SCD-1催化软脂酰CoA形成棕榈油酰CoA, 减少了软脂酰CoA与L-丝氨酸缩合成神经酰胺[12,16,17], 阻止了脂凋亡对肝细胞的损伤. 同时不饱和的十八碳脂肪酸是PPAR a的内源性配体, 通过PPAR a介导增强脂肪酸氧化, 减轻脂肪变性[23].
通过在大鼠普通饲料中添加脂肪, 经一段时间喂养后可成功地塑造非酒精性脂肪肝模型. 高脂饮食引起大鼠血清瘦素水平升高和肝SCD-1mRNA水平下降, 使脂肪在肝内蓄积形成非酒精性脂肪肝. 罗格列酮干预后, 大鼠血清瘦素水平下降和肝SCD-1mRNA水平显著升高, 可减轻因高脂饮食引起的非酒精性肝脏脂肪病变. 瘦素和SCD-1在高脂饮食引起的非酒精性脂肪肝的形成和治疗中起中枢调节作用, 有望成为临床非酒精性脂肪肝的预防和药物开发的一个较为合适的监测指标.
电编: 李琪 编辑:菅鑫妍 审读:张海宁
| 1. | Fan JG, Zhu J, Li XJ, Chen L, Li L, Dai F, Li F, Chen SY. Prevalence of and risk factors for fatty liver in a general population of Shanghai, China. J Hepatol. 2005;43:508-514. [PubMed] [DOI] |
| 3. | Cohen P, Friedman JM. Leptin and the control of metabolism: role for stearoyl-CoA desaturase-1 (SCD-1). J Nutr. 2004;134:2455S-2463S. [PubMed] |
| 4. | Larsson H, Elmståhl S, Berglund G, Ahrén B. Evidence for leptin regulation of food intake in humans. J Clin Endocrinol Metab. 1998;83:4382-4385. [PubMed] [DOI] |
| 5. | Miyazaki M, Kim YC, Ntambi JM. A lipogenic diet in mice with a disruption of the stearoyl-CoA desaturase 1 gene reveals a stringent requirement of endogenous monounsaturated fatty acids for triglyceride synthesis. J Lipid Res. 2001;42:1018-1024. [PubMed] |
| 6. | Attie AD, Krauss RM, Gray-Keller MP, Brownlie A, Miyazaki M, Kastelein JJ, Lusis AJ, Stalenhoef AF, Stoehr JP, Hayden MR. Relationship between stearoyl-CoA desaturase activity and plasma triglycerides in human and mouse hypertriglyceridemia. J Lipid Res. 2002;43:1899-1907. [PubMed] [DOI] |
| 7. | Dobrzyn A, Ntambi JM. The role of stearoyl-CoA desaturase in body weight regulation. Trends Cardiovasc Med. 2004;14:77-81. [PubMed] [DOI] |
| 8. | Cohen P, Ntambi JM, Friedman JM. Stearoyl-CoA desaturase-1 and the metabolic syndrome. Curr Drug Targets Immune Endocr Metabol Disord. 2003;3:271-280. [PubMed] [DOI] |
| 9. | Madiehe AM, Schaffhauser AO, Braymer DH, Bray GA, York DA. Differential expression of leptin receptor in high- and low-fat-fed Osborne-Mendel and S5B/Pl rats. Obes Res. 2000;8:467-474. [PubMed] [DOI] |
| 10. | Ntambi JM, Miyazaki M, Stoehr JP, Lan H, Kendziorski CM, Yandell BS, Song Y, Cohen P, Friedman JM, Attie AD. Loss of stearoyl-CoA desaturase-1 function protects mice against adiposity. Proc Natl Acad Sci USA. 2002;99:11482-11486. [PubMed] [DOI] |
| 11. | Hu CC, Qing K, Chen Y. Diet-induced changes in stearoyl-CoA desaturase 1 expression in obesity-prone and -resistant mice. Obes Res. 2004;12:1264-1270. [PubMed] [DOI] |
| 12. | Unger RH, Orci L. Lipoapoptosis: its mechanism and its diseases. Biochim Biophys Acta. 2002;1585:202-212. [PubMed] [DOI] |
| 13. | Miyazaki M, Kim YC, Gray-Keller MP, Attie AD, Ntambi JM. The biosynthesis of hepatic cholesterol esters and triglycerides is impaired in mice with a disruption of the gene for stearoyl-CoA desaturase 1. J Biol Chem. 2000;275:30132-30138. [PubMed] [DOI] |
| 15. | Harris RB, Mitchell TD, Hebert S. Leptin-induced changes in body composition in high fat-fed mice. Exp Biol Med (Maywood). 2003;228:24-32. [PubMed] |
| 16. | Unger RH, Orci L. Lipotoxic diseases of nonadipose tissues in obesity. Int J Obes Relat Metab Disord. 2000;24 Suppl 4:S28-S32. [PubMed] [DOI] |
| 17. | Kakuma T, Lee Y, Higa M, Wang Zw, Pan W, Shimomura I, Unger RH. Leptin, troglitazone, and the expression of sterol regulatory element binding proteins in liver and pancreatic islets. Proc Natl Acad Sci USA. 2000;97:8536-8541. [PubMed] [DOI] |
| 18. | Cohen P, Miyazaki M, Socci ND, Hagge-Greenberg A, Liedtke W, Soukas AA, Sharma R, Hudgins LC, Ntambi JM, Friedman JM. Role for stearoyl-CoA desaturase-1 in leptin-mediated weight loss. Science. 2002;297:240-243. [PubMed] [DOI] |
| 19. | Heinemann FS, Ozols J. Stearoyl-CoA desaturase, a short-lived protein of endoplasmic reticulum with multiple control mechanisms. Prostaglandins Leukot Essent Fatty Acids. 2003;68:123-133. [PubMed] [DOI] |
| 20. | Miyazaki M, Ntambi JM. Role of stearoyl-coenzyme A desaturase in lipid metabolism. Prostaglandins Leukot Essent Fatty Acids. 2003;68:113-121. [PubMed] [DOI] |
| 21. | Miyazaki M, Jacobson MJ, Man WC, Cohen P, Asilmaz E, Friedman JM, Ntambi JM. Identification and characterization of murine SCD4, a novel heart-specific stearoyl-CoA desaturase isoform regulated by leptin and dietary factors. J Biol Chem. 2003;278:33904-33911. [PubMed] [DOI] |
| 22. | Fan JG, Zhong L, Xu ZJ, Tia LY, Ding XD, Li MS, Wang GL. Effects of low-calorie diet on steatohepatitis in rats with obesity and hyperlipidemia. World J Gastroenterol. 2003;9:2045-2049. [PubMed] [DOI] |
| 23. | Kakuma T, Lee Y, Unger RH. Effects of leptin, troglitazone, and dietary fat on stearoyl CoA desaturase. Biochem Biophys Res Commun. 2002;297:1259-1263. [PubMed] [DOI] |
| 24. | El-Haschimi K, Pierroz DD, Hileman SM, Bjørbaek C, Flier JS. Two defects contribute to hypothalamic leptin resistance in mice with diet-induced obesity. J Clin Invest. 2000;105:1827-1832. [PubMed] [DOI] |
| 25. | Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest. 2002;109:1125-1131. [PubMed] [DOI] |
| 26. | Shimomura I, Shimano H, Korn BS, Bashmakov Y, Horton JD. Nuclear sterol regulatory element-binding proteins activate genes responsible for the entire program of unsaturated fatty acid biosynthesis in transgenic mouse liver. J Biol Chem. 1998;273:35299-35306. [PubMed] [DOI] |
| 27. | Gibbons GF, Wiggins D, Brown AM, Hebbachi AM. Synthesis and function of hepatic very-low-density lipoprotein. Biochem Soc Trans. 2004;32:59-64. [PubMed] [DOI] |
| 28. | Wang H, Storlien LH, Huang XF. Effects of dietary fat types on body fatness, leptin, and ARC leptin receptor, NPY, and AgRP mRNA expression. Am J Physiol Endocrinol Metab. 2002;282:E1352-E1359. [PubMed] [DOI] |
| 29. | Asilmaz E, Cohen P, Miyazaki M, Dobrzyn P, Ueki K, Fayzikhodjaeva G, Soukas AA, Kahn CR, Ntambi JM, Socci ND. Site and mechanism of leptin action in a rodent form of congenital lipodystrophy. J Clin Invest. 2004;113:414-424. [PubMed] [DOI] |
| 30. | Mayerson AB, Hundal RS, Dufour S, Lebon V, Befroy D, Cline GW, Enocksson S, Inzucchi SE, Shulman GI, Petersen KF. The effects of rosiglitazone on insulin sensitivity, lipolysis, and hepatic and skeletal muscle triglyceride content in patients with type 2 diabetes. Diabetes. 2002;51:797-802. [PubMed] [DOI] |
| 31. | Hung YJ, Hsieh CH, Pei D, Kuo SW, Lee JT, Wu LY, He CT, Lee CH, Fan SC, Sheu WH. Rosiglitazone improves insulin sensitivity and glucose tolerance in subjects with impaired glucose tolerance. Clin Endocrinol (Oxf). 2005;62:85-91. [PubMed] [DOI] |