修回日期: 2005-06-27
接受日期: 2005-06-30
在线出版日期: 2005-07-28
目的: 将人胞苷脱氨基酶基因(CD)导入小鼠骨髓细胞后, 观察小鼠对大剂量阿糖胞苷(Ara-C)的耐受性, 探讨骨髓耐受联合化疗的可行性.
方法: 以反转录病毒为载体, 将人胞苷脱氨基酶基因(CD)通过共培养转染入小鼠骨髓干细胞, 观察共培养后的骨髓细胞及受体小鼠骨髓移植后经药物处理后的骨髓细胞耐Ara-C CFU-GM生成情况; 从转基因小鼠骨髓细胞提取DNA, 用PCR检测转基因小鼠骨髓细胞耐药基因的表达; 观察转基因小鼠经大剂量Ara-C化疗后血象、体质量及生存率的变化.
结果: 骨髓移植前共培养后供体的骨髓细胞和骨髓移植后受体含有耐药基因(SFG-CD)的骨髓细胞均有耐药克隆的形成(52%, 54%;与对照组相比χ2分别为124.62, 126.26, P均<0.01), 并明显增加了对Ara-C的耐受性; 与对照组比较含耐药基因组动物经大剂量化疗后, 生存率明显提高(χ2 = 7.42, P<0.01), 血象、体质量下降幅度较小而恢复较快; 转基因小鼠骨髓细胞经PCR检测, 显示有CD基因条带.
结论: 耐药基因可以进入小鼠骨髓细胞并且获得共表达, 提高了造血细胞对Aar-C的耐受性.
引文著录: 路平, 王永来, 金锋, 陈波, 姚凡, 王舒宝, 陈峻青, 徐惠绵, 赵实诚. 胞苷脱氨酶基因对小鼠大剂量化疗的保护作用. 世界华人消化杂志 2005; 13(14): 1705-1708
Revised: June 27, 2005
Accepted: June 30, 2005
Published online: July 28, 2005
AIM: To observe the tolerance to high-dose cytarabine (Ara-C) in mice after the cytidine deaminase (CD) gene is transfected into mouse bone marrow cells, and to explore the feasibility of chemotherapy combined with the tolerance of myelosuppression.
METHODS: Human cytidine deaminase gene was transfected into mouse bone marrow cells by retroviral vector. Then the colony-forming unit granulocyte-macrophage (CFU-GM) was observed in the cells of marrow donor and acceptor mice treated with Ara-C. DNA was extracted from the cells and the drug-resistant genes were detected by polymerase chain reaction (PCR). The blood cell count, weight and survival rate of the mice treated with Ara-C were analyzed.
RESULTS: Drug-resistant colonies appeared both in the bone marrow cells of donor and acceptor mice treated with Ara-C, and the CFU-GMs were 52% and 54% respectively, which were significantly higher than those of the controls (χ2= 124.62, 126.26; both P<0.01). The survival rate was significantly higher in CD-transfected mice as compared with that in the controls (χ2 = 7.42, P<0.01), and the blood cell count and body weight decreased less and recovered sooner. CD gene was expressed in the bone marrow cells of transfected mice.
CONCLUSION: Drug-resistant gene can not only integrate and express in mouse bone marrow cells, but also promote the tolerance to high-dose Ara-C.
- Citation: Lu P, Wang YL, Jin F, Chen B, Yao F, Wang SB, Chen JQ, Xu HM, Zhao SC. Protection of cytidine deaminase gene gainst toxicity of high-dose chemotherapy in mice. Shijie Huaren Xiaohua Zazhi 2005; 13(14): 1705-1708
- URL: https://www.wjgnet.com/1009-3079/full/v13/i14/1705.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v13.i14.1705
骨髓抑制限制了化疗药物的应用, 使化疗药物难以达到治愈或抑制肿瘤生长的剂量, 是影响化疗疗效的关键问题之一. 如何提高骨髓等正常细胞对化疗药物的耐受性, 减少化疗的毒副作用, 已成为肿瘤化疗研究的焦点[1-2]. 我们将胞苷脱氨酶基酶基因(cytidine deaminase, CD)导入小鼠骨髓细胞中, 观察小鼠对大剂量阿糖胞苷(cytosine arabinoside, Ara-C)的耐受性, 结果如下.
含耐药基因的反转录病毒载体SFG-CD(含人突变的胞嘧啶脱氨酶mCD), 转染入病毒产生细胞(amph-otropic packaging cell, GP-Am12, 简称Am12), 构成AM12-SFG-CD, 简称为SFG-CD. 以上载体及细胞系由赵实诚教授提供. 动物为BALB/c雄性小鼠8-10周龄, 中国医科大学实验动物部提供, 许可证号:SCXK(辽)2003-0009.IMDM(Iscove's Modified Dulbecco's Medium)培养液, 美国Gibco公司产品; 含小鼠造血增殖因子条件培养液培养液(WEHI), 美国Gibco公司产品.
1.2.1小鼠处理: 小鼠行5-FU尾静脉注射(150 mg/kg), 该药可杀伤成熟粒细胞而不影响造血祖细胞, 注射4 d后取其股骨、胫骨的骨髓细胞(2-5)×107, 制成单细胞悬液. 计数骨髓有核细胞总数, 使骨髓有核细胞和转基因之病毒产生细胞按1:1的比例, 置于含200 mL/L小牛血清和10 g/L谷氨酰胺, 100 g/L WEHI中, 分两组(SFG-CD, Am12)在37 ℃ 50 mL/L CO2条件下共培养48 h. 含耐药基因病毒产生细胞系在共培养前2 h照射1.5 Gy. 共培养后收集骨髓细胞, 制成细胞悬液(在PBS平衡液中作为骨髓移植及CFU-GM培养用). 受体小鼠在骨髓移植前均经8 Gy致死量照射, 以完全破坏其自身造血功能, 观察被输入的造血细胞在体内增殖及血液的耐药情况. 12只小鼠根据体质量随机分为两组:A组转染SFG-CD;B组阴性对照组, 被输入的骨髓细胞仅与Am12共培养不含耐药基因. 每只小鼠根据其组别, 在致死量照射后12 h尾静脉注射2×102骨髓细胞. 骨髓移植后4 wk每只小鼠ip Ara-C(30 mg/kg)连续4 d. 期中及注射后(25, 30, 35, 40及45 d)观察血象、体质量及生存率的变化.
检测共培养后的骨髓细胞行体外实验, 受体小鼠骨髓移植和药物处理45 d后取骨髓细胞行体内实验. 培养分为两组:A组加Ara-C, 终浓度为500 nmol/L;B组为对照组, 不加药物. 每组共6 mL IMDM培养液含骨髓细胞3×106, 平分为3个平皿进行培养, 含10 g/L甲基纤维素, 100 mL/L胎牛血清, 150 g/L WEHI培养液, 10 g/L碳酸氢钠, 10 g/L丙酮酸钠, 10 g/L必需与非必需氨基酸, 5 g/L多种维生素及100 kU/L青链霉素等, 胎牛血清在加入混合培养液前经用1 mg/L胸苷酸磷酶37 ℃1 h处理. 培养皿置含50 mL/L CO2 37 ℃培养箱7-10 d后计数集落, 进行结果分析.
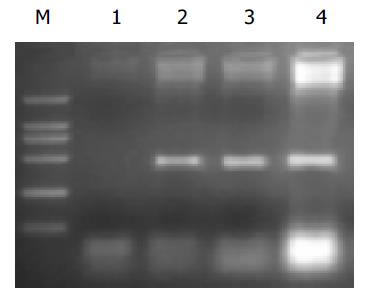
取经基因转染及药物处理后小鼠骨髓细胞, 按饱合氯化钠法提取DNA.50 mL的PCR反应体系终浓度为0.125 mmol/L dNTPs. 两种引物0.5 mmol/L, 0.05 MU/L Taq DNA聚合酶, 模板DNA 150 ng.PCR扩增条件是:94 ℃变性10 min, 94 ℃变性1 min, 58 ℃退火1 min, 72 ℃延伸2 min, 共40循环之后72 ℃延伸10 min.PCR产物在0.8 g/L琼脂糖凝胶中进行电泳, 照像分析. 检测该试验耐药基因CD的引物序列(扩增产物为465 bp): 上游5'-ACG GGATCCATGGCCCAGAAGCGTCCTG-3', 下游5'-CCGCTCG AGTCACTGAGTCTTCTGCAG-3'.
统计学处理 生存分析应用Log Rank检验(SPSS10.0统计软件), 计数资料行χ2检验, 计量资料经t检验. P<0.05为差异有统计学意义.
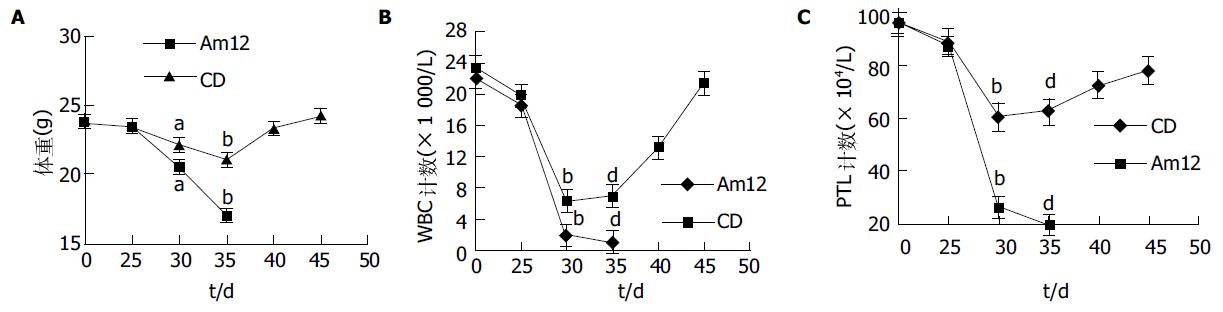
小鼠注射Ara-C后, 转染耐药基因的动物与对照相比, 动物存活率明显提高、体质量、白细胞和血小板恢复较快. 转染耐药基因后CD组小鼠在大剂量Ara-C处理后45 d存活率达67%(4/6), 对照组无存活, 经Log Rank检验χ2 = 7.42, P<0.01. 小鼠注射Ara-C后, 体质量、白细胞(WBC)和血小板(PLT)均开始下降, CD组小鼠血象、体质量下降幅度较小而恢复较快, 45 d体质量、白细胞和血小板均恢复正常(图1A-C).
骨髓移植前供体的骨髓细胞和骨髓移植后受体的骨髓细胞含有耐药基因组均有耐药克隆的形成(表1). 骨髓移植前对照组注射终浓度为500 nmol/L的Ara-C后, 对药物的耐药克隆分别为52%和9%, 骨髓移植后耐药克隆分别为54%和7%. 转基因小鼠骨髓细胞提取的DNA, 经PCR检测, 显示有CD基因条带, 说明动物耐药基因转染已得到分子生物学表达(图2).
逆转录病毒(retrovirus, RV)是目前基因治疗应用较多的载体. RV 是单链RNA病毒, 主要特点是[3-4]: (1)临床研究具有相对的安全性, 未发生严重的不良结果; (2)外源基因能稳定地整合入靶细胞内; (3)可携带7-8 kb的外源基因片段; (4)只转染分裂期细胞; (5)转染靶细胞范围广; (6)转移效率较高. 其不足之处是: (1)插入外源基因较小, 难以满足较大基因的转移; (2)只适于转染分裂期细胞; (3)病毒滴度是限制临床应用的一个主要方面. 决定基因治疗疗效的关键为: 目的基因的导入, 并在靶细胞表达, 及其表达的可调控性.
阿糖胞苷(Ara-C)是临床治疗乳腺癌、白血病、淋巴瘤等多种恶性肿瘤的常用化疗药物. 但在治疗过程中其大剂量应用产生的骨髓抑制等毒副作用较严重, 限制了其疗效. 胞苷脱氨酶(CD)是阿糖胞苷(Ara-C)类化疗药细胞内代谢的水解酶[5].Ara-C通过核苷载体进入细胞后经脱氧胞苷激酶(DCK)磷酸化生成Ara-CMP, 再两步磷酸化生成活性产物Ara-CTP, 同时经CD迅速脱氨为相对无活性产物阿糖尿苷(Ara-U), Ara-CMP经5'核苷酸酶(5'NU)去磷酸化[6]. 目前研究认为, Ara-C耐药机制主要与转运摄取过程障碍和胞内活化分解酶异常相关, 如DCK活性降低或缺乏, CD和5'NU活性增加. CD主要功能是在DNA嘧啶核苷代谢中催化生理性胞苷和脱氧胞苷水解脱氨生成尿苷和脱氧尿苷, 同时也催化抗肿瘤药物中核苷类似物脱氨生成无活性的代谢产物, 如催化Ara-C生成无活性的Ara-U[7].
我们已报道用逆转录病毒载体将CD基因转导小鼠骨髓细胞, 在体外实验观察到CD基因可以进入小鼠骨髓细胞并且获得共表达, 提高了造血细胞对Aar-C的耐药性[8]. 为进一步研究CD耐药基因治疗体内实验的可行性, 了解导入基因在小鼠体内的表达, 对骨髓细胞的保护作用资料等, 本实验以反转录病毒为载体, 将人CD通过共培养转染入小鼠骨髓干细胞, 观察到受体小鼠骨髓细胞均有耐药克隆的形成, 并明显增加了对Ara-C的耐受; 与对照组比较含耐药基因组动物经大剂量化疗后, 生存率明显提高(P<0.01), 血象逐渐恢复正常; 转基因小鼠骨髓细胞经PCR检测, 显示有CD基因条带; 耐药基因转染后小鼠骨髓对Ara-C的耐受明显增加. 因此, 我们认为逆转录病毒CD基因转导对小鼠骨髓有化疗保护作用, 本实验为临床超剂量化疗以求最大限度杀伤肿瘤细胞提供了一个较好的动物模型, 可能为提高肿瘤的化疗疗效提供帮助.
编辑: 张海宁
| 1. | Takebe N, Zhao SC, Ural AU, Johnson MR, Banerjee D, Diasio RB, Bertino JR. Retroviral transduction of human dihydrop-yrimidine dehydrogenase cDNA confers resistance to 5-fluorouracil in murine hematopoietic progenitor cells and human CD34+-enriched peripheral blood progenitor cells. Cancer Gene Ther. 2001;8:966-973. [PubMed] [DOI] |
| 2. | Belur LR, Boelk-Galvan D, Diers MD, McIvor RS, Zimmerman CL. Methotrexate accumulates to similar levels in animals transplanted with normal versus drug-resistant transgenic marrow. Cancer Res. 2001;61:1522-1526. [PubMed] |
| 3. | Takebe N, Xu LC, MacKenzie KL, Bertino JR, Moore MA. Methotrexate selection of long-term culture initiating cells following transduction of CD34 (+) cells with a retrovirus containing a mutated human dihydrofolate reductase gene. Cancer Gene Ther. 2002;9:308-320. [PubMed] [DOI] |
| 4. | Capiaux GM, Budak-Alpdogan T, Takebe N, Mayer-Kuckuk P, Banerjee D, Maley F, Bertino JR. Retroviral transduction of a mutant dihydrofolate reductase-thymidylate synthase fusion gene into murine marrow cells confers resistance to both methotrexate and 5-fluorouracil. Hum Gene Ther. 2003;14:435-446. [PubMed] [DOI] |
| 5. | Eliopoulos N, Al-Khaldi A, Beausejour CM, Momparler RL, Momparler LF, Galipeau J. Human cytidine deaminase as an ex vivo drug selectable marker in gene-modified primary bone marrow stromal cells. Gene Ther. 2002;9:452-462. [PubMed] [DOI] |
| 6. | Gourdeau H, Bibeau L, Ouellet F, Custeau D, Bernier L, Bowlin T. Comparative study of a novel nucleoside analogue (Troxatyl, troxacitabine, BCH-4556) and AraC against leukemic human tumor xenografts expressing high or low cytidine deaminase activity. Cancer Chemother Pharmacol. 2001;47:236-240. [PubMed] [DOI] |
| 7. | Beausejour CM, Eliopoulos N, Momparler L, Le NL, Momparler RL. Selection of drug-resistant transduced cells with cytosine nucleoside analogs using the human cytidine deaminase gene. Cancer Gene Ther. 2001;8:669676. [PubMed] [DOI] |