修回日期: 2005-04-08
接受日期: 2005-04-09
在线出版日期: 2005-06-28
目的: 探讨益肝康及其拆方丹参小复方(丹参、黄芪、归尾)对肝星状细胞PDGF与受体表达的影响.
方法: 分别制备益肝康及丹参小复方纯化浸膏、正常大鼠及肝纤维化大鼠药物血清, 温育体外培养的HSC. RT-PCR和Western blot技术检测PDGF-BB mRNA及蛋白表达, RT-PCR和流式细胞技术检测PDGFR-b mRNA及蛋白表达.
结果: 益肝康及丹参小复方纯化浸膏使PDGF-BB蛋白表达分别下调了78.79%、56.77%(P<0.01), 使其mRNA表达分别下调了67.13%、43.59%(P<0.01), 使PDGFR-b蛋白表达分别下调了11.61%、6.79%(P<0.01), 使其mRNA表达分别下调了54.85%、30.51%(P<0.01); 正常大鼠益肝康及丹参小复方药物血清使PDGF-BB蛋白表达分别下调了68.05%、49.95%(P<0.01), 使其mRNA表达分别下调了55.87%、38.66%(P<0.01), 使PDGFR-b蛋白表达分别下调了10.98%、6.48%(P<0.01), 使其mRNA表达分别下调了39.67%、21.38%(P<0.01); 肝纤维化大鼠益肝康及丹参小复方药物血清使PDGF-BB蛋白表达分别下调了81.93%、67.31%(P<0.01), 使其mRNA表达分别下调了72.68%、55.49%(P<0.01), 使PDGFR-b蛋白表达分别下调了17.43%、11.15%(P<0.01), 使其mRNA表达分别下调了60.46%、46.47%(P<0.01), 全方作用优于拆方(P<0.01), 肝纤维化大鼠药物血清作用优于正常大鼠药物血清(P<0.01).
结论: 益肝康及丹参小复方可显著抑制PDGF与PDGFR-b基因和蛋白表达, 此可能是该方抗肝纤维化的主要机制之一, 且全方作用优于拆方.
引文著录: 姚希贤, 房红梅, 王军民. 拆方益肝康与药物血清对肝星状细胞PDGF及受体表达的影响. 世界华人消化杂志 2005; 13(12): 1412-1416
Revised: April 8, 2005
Accepted: April 9, 2005
Published online: June 28, 2005
AIM: To investigate the effects of Yigankang and small compound of radix salviae militiorrhizae on protein and gene expression of platelet-derived growth factor-BB (PDGF-BB) and platelet-derived growth factor receptor-b (PDGFR-b) in hepatic stellate cells (HSCs).
METHODS: The purified extracts and pharmaceutical serum from normal and liver fibrosis rats of Yigankang and small compound of radix salviae militiorrhizae were prepared respectively. The expression of PDGF-BB mRNA and protein were detected by reverse transcription polymerase chain reaction (RT-PCR) and Western blot, and the expression of PDGFR-b mRNA and protein were measured by RT-PCR and flow cytometry.
RESULTS: The purified extracts of Yigankang and small compound of radix salviae militiorrhizae decreased PDGF-BB protein expression by 78.79% and 56.77% and its mRNA by 67.13% and 43.59% (P<0.01); lowered PDGFR-b protein expression by 11.61% and 6.79% and its mRNA by 54.85% and 30.51% respectively (P<0.01). Yigankang and small compound of radix salviae militiorrhizae pharmaceutical serum from normal rats decreased PDGF-BB protein expression by 68.05% and 49.95% and its mRNA by 55.87% and 38.66% (P<0.01); lowered PDGFR-b protein expression by 10.98% and 6.48% and its mRNA by 39.67% and 21.38% respectively (P<0.01). Yigankang and small formula of radix salviae militiorrhizae pharmaceutical serum from liver fibrosis rats reduced PDGF-BB protein expression by 81.93% and 67.31% and its mRNA by 72.68% and 55.49% (P<0.01); decreased PDGFR-b protein expression by 17.43% and 11.15% and its mRNA by 60.46% and 46.47% respectively (P<0.01). With respect to the inhibitory effects mentioned above, Yigankang had more evidently action than its separated recipe did, and the pharmaceutical serum from liver fibrosis rats was superior to that from normal rats.
CONCLUSION: The inhibitory effect of Yigankang and its separated recipe on PDGF-BB and PDGFR-b expression may be one of the main mechanisms of their antifibrotic action, and the whole recipe was superior to the separated recipe.
- Citation: Yao XX, Fang HM, Wang JM. Effects of Yigankang and its separated recipe on expression of platelet-derived growth factor and its receptor in hepatic stellate cells. Shijie Huaren Xiaohua Zazhi 2005; 13(12): 1412-1416
- URL: https://www.wjgnet.com/1009-3079/full/v13/i12/1412.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v13.i12.1412
以丹参等活血化瘀药为主组方的益肝康有较好的抗肝纤维化作用[1-12].为进一步了解该方的抗肝纤维化作用机制, 我们采用对比血清药理学方法, 即分别提取正常大鼠和CCl4造模肝纤维化大鼠益肝康及其拆方丹参小复方(丹参、黄芪、归尾)的药物血清, 并同时设立生药纯化浸膏进行对照, 作用于体外培养的肝星状细胞(hepatic stellate cell, HSC), 探讨该方对HSC血小板衍生生长因子(platelet-derived growth factor, PDGF)及其受体(platelet-derived growth factor receptor, PDGFR)基因与蛋白表达的影响, 以探讨新的药物作用靶点, 并籍药物血清体外实验尽量准确再现在体药物分别与正常及病态机体相互作用的过程.
健康雄性SD大鼠, 清洁级, 体质量300-400 g, 购自河北医科大学实验动物中心.益肝康由丹参、黄芪、归尾、赤芍等组成, 丹参小复方由丹参、黄芪、归尾三味药组成, 拆方组中药物用量与全方组单味药用量一致, 本室自行制备成浓缩水煎剂灌胃液, 每毫升含生药0.72 g.HSC株CFSC由美国Greenwel教授建株并惠赠, 表型为活化的HSC.兔抗鼠PDGF-BB多克隆抗体、兔抗鼠PDGFR-b多克隆抗体、兔抗鼠Actin多克隆抗体均购自武汉博士德生物工程有限公司, HRP标记的山羊抗兔IgG、ECL发光试剂盒、浓缩型DAB试剂盒均购自北京中山生物技术有限公司, 蛋白marker购自北京鼎国生物技术发展中心, 硝酸纤维素膜与DNA marker购自华美生物工程公司, RT-PCR扩增系统购自美国Promega公司, 总RNA提取试剂盒购自北京博大泰克生物技术有限公司, PDGF-BB及PDGFR-b引物由上海生工生物工程公司合成, 其余试剂均为进口或国产分析纯.
1.2.1 药物血清制备: 雄性SD大鼠90只, 随机分为甲组(正常大鼠药物血清组)和乙组(肝纤维化大鼠药物血清组), 每小组45只, 乙组大鼠给予400 mL/L CCl4纯制花生油溶液sc, 首剂4 mL/kg, 以后改为2 mL/kg, 2次/wk, 共9 wk; 甲组大鼠给予纯制花生油sc, 剂量及时间同乙组.将两组大鼠各随机分为3小组, 每组15只, 甲1/乙1: 对照组, 甲2/乙2: 丹参小复方组, 甲3/乙3: 益肝康组, 药物组按成人每kg体质量10倍用量灌服益肝康/丹参小复方水煎剂, 每日2次, 连续5 d, 对照组灌以生理盐水.第6 d按常规量再灌胃1次, 1 h后, 下腔静脉取血, 静置3 h, 3 000 r/min低温离心20 min, 分离血清, 56℃灭活30 min, 过滤除菌, 加入RPMI1640培养液, 分别配制成含100 mL/L药物/对照血清的培养基.
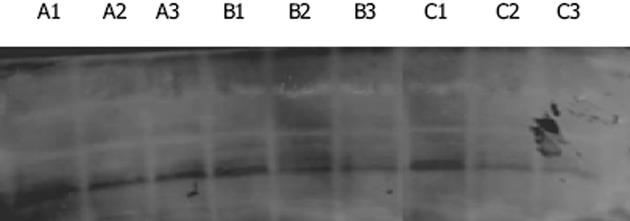
1.2.2 体外实验: 每次实验均在呈指数生长的细胞中进行, 接种2.0×105-3.0×105个细胞于25 cm2培养瓶, 孵育至细胞近100%融合时, 换不含胎牛血清的培养液继续培养24 h, 使细胞基本同步化于G0期后, 分别添加生药及药物/对照血清, 温育24 h, 进行下一步实验.分组如下: A组: 生药纯化浸膏组(A1: 正常对照组; A2: 104 g/L丹参小复方组; A3: 104 g/L益肝康组); B组: 100 mL/L正常大鼠药物血清组(B1: 对照组; B2: 丹参小复方组; B3: 益肝康组); C组: 100 mL/L肝纤维化大鼠药物血清组(C1: 对照组; C2: 丹参小复方组; C3: 益肝康组), 每组6瓶.PDGF-BB蛋白测定采用Western blot方法.提取HSC胞质蛋白, 进行100 g/L聚丙烯酰胺凝胶电泳; 冷却状态下转膜, 170 mA, 恒流, 1 h; 1∶100兔抗PDGF-BB多克隆抗体4℃密封过夜; 1∶1 000辣根过氧化物酶标记的山羊抗兔IgG室温密封摇动孵育2 h; ECL发光剂显色1 min, X光片暗室曝光30 s至数分钟, 显影、定影, 密度扫描分析, 灰度值以PDGF-BB与Actin的积分吸光度的比值表示.PDGFR-b蛋白测定 采用流式细胞技术进行检测.收集细胞, 调整细胞浓度不少于1×109/L, 700 mL/L乙醇4℃固定过夜, PBS洗2遍, 1∶100兔抗PDGFR-b多克隆抗体室温孵育30 min, 羊抗兔FITC-IgG二抗工作液避光室温孵育30 min, PBS洗涤, 离心, 1 000 r/min×5 min, 弃上清, 加入PBS 0.1 mL, 500目铜网过滤后上机检测.使用美国FACS-420流式细胞仪检测, 设PBS代替一抗和二抗的阴性对照, 以及只加一抗或二抗的阳性对照, 蛋白定量以mode值表示.PDGF-BB及PDGFR-b mRNA测定采用RT-PCR方法.提取HSC总RNA, 进行完整性鉴定及纯度和定量检测, 取2 mg总RNA进行RT-PCR, 使用50 mL反应体系分别扩增PDGF-BB和b-actin, PDGFR-b和b-actin.PDGF-BB引物序列: 上游5'-CTGAGCTGGACTTGAACATGA-3', 下游5'-CACTACTGTCTCACACTTGCAGG-3'(扩增产物大小为380 bp), PDGFR-b: 上游5'-TCGTCCTCAACATTTCGAGC-3', 下游5'-TCATAGGGTACATGTAGGGGGAT-3'(扩增产物大小为397 bp), b-actin: 上游5'-GTCACCCACACT
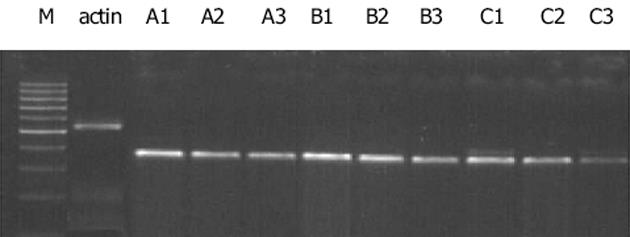
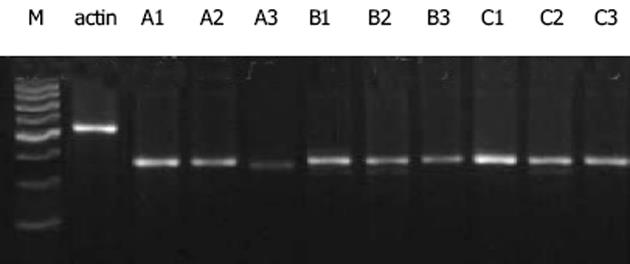
GTGCCCATCT-3', 下游5'-ACAGAGTACTTGCGCTCAGGAG-3'(扩增产物大小为542 bp), 具体循环参数如下, RT: 41℃, 45 min; PCR: 94℃预变性5 min进入循环, 94℃变性45 s, 55℃退火30 s, 72℃延伸1 min, 经35个循环扩增后72℃延伸7 min.取10 mL RT-PCR产物及10 mL DNA marker进行15 g/L琼脂糖凝胶电泳, 采用MULIT IMAGE凝胶图像分析仪进行吸光度扫描, 结果以PDGF-BB/PDGFR-b与b-actin的积分吸光度的比值表示.
统计学处理 实验数据以mean盨D表示, 组间比较采用单因素方差分析, 应用最小显著差法(LSD)进行两两比较.所有资料均用计算机统计软件SPSS11.5进行统计分析, 以P<0.01为显著性差异.
经益肝康及丹参小复方浸膏(104 g/L)干预后, PDGF-BB蛋白表达均较对照组显著减少(A3: 0.315±0.096, A2: 0.642±0.127 vs A1: 1.485±0.150, P<0.01); 全方对PDGF-BB蛋白表达的抑制作用强于拆方(P<0.01).两种方法制备的100 mL/L益肝康及丹参小复方药物血清均可显著抑制PDGF-BB蛋白表达(B3: 0.502±0.100, B2: 0.793±0.158 vs B1: 1.566±0.212, P<0.01; C3: 0.317±0.008, C2: 0.524±0.109 vs C1: 1.603±0.125, P<0.01), 全方作用优于拆方(P<0.01), 肝纤维化大鼠药物血清组作用优于正常大鼠药物血清组(P<0.01)(图1).
经益肝康及丹参小复方浸膏(104 g/L)干预后, PDGF-BB基因表达均较对照组显著减少(A3: 0.328±0.040, A2: 0.563±0.037 vs A1: 0.998±0.042, P<0.01); 全方对PDGF-BB基因表达的抑制作用强于拆方(P<0.01).两种方法制备的100 mL/L 益肝康及丹参小复方药物血清均可显著抑制PDGF-BB基因表达(B3: 0.435±0.038, B2: 0.604±0.041 vs B1: 0.984±0.047, P<0.01; C3: 0.301±0.030, C2: 0.489±0.045 vs C1: 1.097±0.051, P<0.01), 全方作用优于拆方(P<0.01), 肝纤维化大鼠药物血清组作用优于正常大鼠药物血清组(P<0.01)(图2).
经益肝康及丹参小复方浸膏(104 g/L)干预后, PDGFR-b蛋白表达均较对照组显著减少(A3: 4.95±0.095, A2: 5.22±0.143 vs A1: 5.60±0.100, P<0.01); 全方对PDGFR-b蛋白表达的抑制作用强于拆方(P<0.01).两种方法制备的100 mL/L益肝康及丹参小复方药物血清均可显著抑制PDGFR-b蛋白表达(B3: 5.09±0.120, B2: 5.30±0.109 vs B1: 5.66±0.136, P<0.01; C3: 4.81±0.062, C2: 5.18±0.117 vs C1: 5.82±0.155, P<0.01), 全方作用优于拆方(P<0.01), 肝纤维化大鼠药物血清组作用优于正常大鼠药物血清组(P<0.01).
经益肝康及丹参小复方浸膏(104 g/L)干预后, PDGFR-b基因表达均较对照组显著减少(A3: 0.410±0.027, A2: 0.631±0.032 vs A1: 0.908±0.035, P<0.01), 全方对PDGFR-b基因表达的抑制作用强于拆方(P<0.01).两种方法制备的100 mL/L益肝康及丹参小复方药物血清均可显著抑制PDGFR-b基因表达(B3: 0.555±0.030, B2: 0.724±0.035 vs B1: 0.920±0.038, P<0.01; C3: 0.382±0.029, C2: 0.517±0.033 vs C1: 0.965±0.040, P<0.01), 全方作用优于拆方(P<0.01), 肝纤维化大鼠药物血清组作用优于正常大鼠药物血清组(P<0.01)(图3).
HSC是肝纤维化形成的中心环节与细胞学基础, 此在近年来国内外研究业已取得广泛共识[13-15].细胞因子是细胞间信号转导、联系的重要物质基础, HSC的活化过程中始终贯穿着转化生长因子、血管内皮生长因子、白介素-6等细胞网络因子及其受体的变化[16-19].PDGF是目前已知的最强的促HSC分裂增殖因子, 与细胞膜上的相应受体结合后发挥其生物学效应.PDGFR由a、b两种亚单位以二硫键连接形成, 其与PDGF结合力相差很大, a单位与PDGFA链及B链均有较高亲和力, 而b亚单位仅与B链有高亲和力.在肝纤维化时, HSC表面的PDGFR以b受体为主, 与PDGF-BB具有较强的亲和力, 认为PDGF-BB以及PDGFR-b在肝纤维化过程中的作用尤为突出[20-31].HSC在体外无包被的塑料培养皿中培养, 可自发向肌成纤维样细胞转化, 具有体内活化的生物学特征[32].HSC株CFSC从CCl4诱发的肝硬化大鼠中分离并通过培养使细胞自发获得永久性, 表型为活化的HSC, 是较为理想的肝纤维化研究模型[33].本研究分别采用益肝康与拆方的纯化浸膏、正常大鼠及肝纤维化大鼠药物血清进行体外HSCs培养, 观察其对肝纤维化形成与发展过程中最重要的细胞因子之一-PDGF与受体蛋白及基因表达的作用情况, 结果表明益肝康与丹参小复方的纯化浸膏及药物血清均具有良好的抑制PDGF与PDGFR-b基因和蛋白表达的作用.而且药物配伍后的全方组(A3, B3, C3)对HSC PDGF与PDGFR-b基因及蛋白表达较拆方组(A2, B2, C2)具有更为显著的抑制作用, 充分体现了符合中医基础理论君臣佐使配方组合的优势.
中药复方益肝康(前身为益肝冲剂, 于1980年投产并应用于临床, 益肝康为研制者对该方进一步研究改进而成)系重用丹参, 辅以当归、赤芍等活血化瘀, 并用黄芪等益气健脾药而成.为明确有效方剂益肝康抗肝纤维化的作用机制, 以及为得到专一性更强、疗效更显著的新药, 本研究所长期以来围绕肝纤维化的主要病理环节对该复方有关单味药、拆方小复方及全方进行了大量临床与实验研究, 结果表明, 该药具有消除症状, 改善肝功能, 减少胶原沉积作用; 可抑制HSCs活化、增殖, 促进HSCs凋亡, 并有抗脂质过氧化, 保护受损肝细胞及线粒体等细胞器功能, 而且无明显毒副作用[1-7].本研究表明益肝康、丹参小复方的纯化浸膏及药物血清均具有良好的抑制PDGF与PDGFR-b基因和蛋白表达的作用, 说明该复方作用机制之一是从mRNA和蛋白水平抑制PDGF及PDGFR-b表达, 从而抑制HSC的激活、增殖以及细胞外基质的合成, 发挥抗肝纤维化作用.
药物血清中发挥作用的有效物质包括母体药物、药物在机体内的代谢物以及药物诱生的内源性物质, 本部分实验采用直接加药法与整体动物给药、分离药物血清作用于体外培养HSC的血清药理学方法进行对比研究, 结果表明益肝康、丹参小复方的纯化浸膏及药物血清均具有良好抑制PDGF与PDGFR-b基因和蛋白表达的作用, 推测该复方中真正起到抑制PDGF与PDGFR-b表达作用的有效物质可能仍以原药成分为主.本研究结果表明肝纤维化大鼠药物血清对HSC PDGF与PDGFR-b基因及蛋白表达的抑制作用优于正常大鼠药物血清, 分析原因, 是否与肝纤维化大鼠肝功能下降, 对药物的转化、代谢能力降低, 从而导致病态机体内药物有效成分浓度提高, 作用时间延长, 使抗肝纤维化作用增强有关?抑或中医辨证论治的特点使得该方对肝纤维化病态机体更具针对性, 疗效更明显.血清药理学的出现为科学阐明中药复方的有效成分及作用机制提供了一种有力的工具, 但作为一种新兴的实验方法, 在实验技术上还需要进一步探讨、规范和完善.
编辑: 潘伯荣 审读: 张海宁
| 2. | Yao XX, Tang YW, Yao DM, Xiu HM. Effects of Yigan Decoction on proliferation and apoptosis of hepatic stellate cells. World J Gastroenterol. 2002;8:511-514. [PubMed] [DOI] |
| 3. | 姚 希贤, 姚 欣, 修 贺明, 孙 泽明, 宋 梅, 冯 丽英. "益肝浓缩煎剂"等活血化瘀药抗大鼠肝纤维化作用实验研究. 胃肠病学和肝病学杂志. 2001;10:217-222. |
| 4. | Yao XX, Cui DL, Sun YF, Feng LY, Sun ZM, Song M. Study on the anti-liver fibrosis effect of benefit liver granule and its mechanism in rats. Chin J Integr Med. 2002;8:118-121. |
| 10. | 唐 有为, 姚 希贤, 姚 洪森. 益肝康对实验性肝纤维化大鼠肝细胞的保护作用及超微结构观察. 中国中西医结合消化杂志. 2002;10:76-78. |
| 13. | Issa R, Williams E, Trim N, Kendall T, Arthur MJ, Reichen J, Benyon RC, Iredale JP. Apoptosis of hepatic stellate cells involvement in resolution of biliary fibrosis and regulation by soluble growth factors. Gut. 2001;48:548-557. [PubMed] [DOI] |
| 14. | Fisher R, Schmitt M, Bode JG, Haussinger D. Expression of the peripheral-type benzodiazepine receptor and apoptosis induction in hepatic stellate cells. Gastroenterology. 2001;120:1212-1226. [PubMed] [DOI] |
| 15. | Nagler A, Pines M, Abadi U, Pappo O, Zeira M, Rabbani E, Engelhardt D, Ohana M, Chowdhury NR, Chowdhury JR. Oral tolerization ameliorates liver disorders associated with chronic graft versus host disease in mice. Hepatology. 2000;31:641-648. [PubMed] [DOI] |
| 16. | Paradis V, Dargere D, Bonvoust F, Vidaud M, Segarini P, Bedossa P. Effects and regulation of connective tissue growth factor on hepatic stellate cells. Lab Invest. 2002;82:767-774. [PubMed] [DOI] |
| 17. | Pinzani M, Marra F. Cytokine receptors and signaling in hepatic stellate cells. Semin Liver Dis. 2001;21:397-416. [PubMed] [DOI] |
| 18. | Li D, Friedman SL. Liver fibrogenesis and the role of hepatic stellate cells: New insights and prospects for therapy. J Gastroenterol Hepatol. 1999;4:618-633. [PubMed] [DOI] |
| 19. | Friedman SL. Cytokines and fibrogenesis. Semin Liver Dis. 1999;19:129-140. [PubMed] [DOI] |
| 20. | Pinzani M. PDGF and signal transduction in hepatic stellate cells. Front Biosci. 2002;7:1720-1726. [PubMed] [DOI] |
| 21. | Ikura Y, Morimoto H, Ogami M, Jomura H, Ikeoka N, Sakurai M. Expression of platelet-derived growth factor and its receptor in livers of patients with chronic liver disease. J Castroentrol. 1997;32:496-501. [PubMed] [DOI] |
| 22. | Pinani M, Milani S, Herbst H, DeFranco R, Grappone C, Gentilini A, Caligiuri A, Pellegrini G, Ngo DV, Romanelli RG. Expression of platelet-derived growth factor and its receptors in normal human liver and during active hepatic fibrosis. Am J Pathol. 1996;148:785-800. [PubMed] |
| 23. | Marra F, Gentilini A, Pinzani M, Choudhury GG, Parola M, Herbst H, Dianzani MU, Laffi G, Abboud HE, Gentilini P. Phosphatidylinositol 3-kinase is required for platelet-derived growth factor's actions on hepatic stellate cells. Gastroenterology. 1997;112:1297-1306. [PubMed] [DOI] |
| 24. | Isbrucker RA, Oeferson TC. platelet-derived growth factor and pentoxifylline modulation of collagen synthesis in myofibroblasts. Toxicology and Applied. Pharmacology. 1998;149:120-126. [PubMed] [DOI] |
| 25. | Herbst H, Schupan D, Milani S. Fibrogenesis and fibrolysis in the liver. Verh Dtsch Ges Pathol. 1995;79:15-27. [PubMed] |
| 26. | Wong L, Yamasaki G, Johnson RJ, Friedman SL. Induction of b-platelet-derived growth factor receptor in rat hepatic lipocytes during cellular activation in vivo and in culture. J Clin Invest. 1994;94:1563-1569. [PubMed] [DOI] |
| 27. | Di Sario A, Bendia E, Svegliati-Baroni G, Marzioni M, Ridolfi F, Trozzi L, Ugili L, Saccomanno S, Jezequel AM, Benedetti A. Rearrangement of the cytoskeletal network induced by platelet-derived growth factor in rat hepatic stellate cells: role of different intracellular signalling pathways. J Hepatol. 2002;36:179-190. [PubMed] [DOI] |
| 28. | Kinnman N, Goria O, Wendum D, Gendron MC, Rey C, Poupon R, Housset C. Hepatic stellate cell proliferation is an early platelet-derived growth factor-mediated cellular event in rat cholestatic liver injury. Lab Invest. 2001;81:1709-1716. [PubMed] [DOI] |
| 29. | Okuyama H, Shimahara Y, Kawada N, Seki S, Kristensen DB, Yoshizato K, Uyama N, Yamaoka Y. Regulation of cell growth by redox-mediated extracellular proteolysis of platelet-derived growth factor receptor beta. J Biol Chem. 2001;276:28274-28280. [PubMed] [DOI] |
| 30. | Taylor CC. Platelet-derived growth factor activates porcine thecal cell phosphatidylinositol-3-kinase-Akt/PKB and ras-extracellular signal-regulated kinase-1/2 kinase signaling pathways via the platelet-derived growth factor-beta receptor. Endocrinology. 2000;141:1545-1553. [PubMed] [DOI] |
| 31. | Marra F, Arrighi MC, Fazi M, Caligiuri A, Pinzani M, Romanelli RG, Efsen E, Laffi G, Gentilini P. Extracellular signal-regulated kinase activation differentially regulates platelet-derived growth factor's actions in hepatic stellate cells, and is induced by in vivo liver injury in the rat. Hepatology. 1999;30:951-958. [PubMed] [DOI] |
| 32. | Friedman SL. Cellular sources of collagen and regulation of collegen production in liver. Sem in Liver Dis. 1990;10:20-29. [PubMed] [DOI] |
| 33. | Greenwel P, Schwartz M, Rossas M, Peyrol S, Grimaud JA, Rojkind M. Characterization of fat-storing cell lines derived from normal and CCl4-cirrhotic livers. Lab Invest. 1991;65:644-653. [PubMed] |