修回日期: 2005-04-01
接受日期: 2005-04-08
在线出版日期: 2005-06-28
目的: 探讨孕鼠肝内胆汁淤积症对肝脏和胎盘的毒性作用.
方法: 应用雌孕激素建立妊娠肝内胆汁淤积大鼠模型, 光镜和电镜观察母鼠肝脏和胎盘的病理改变.
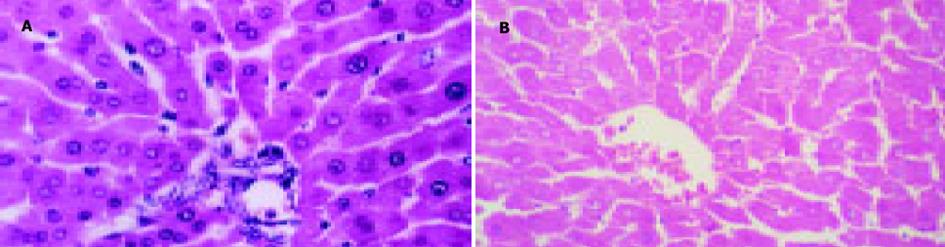
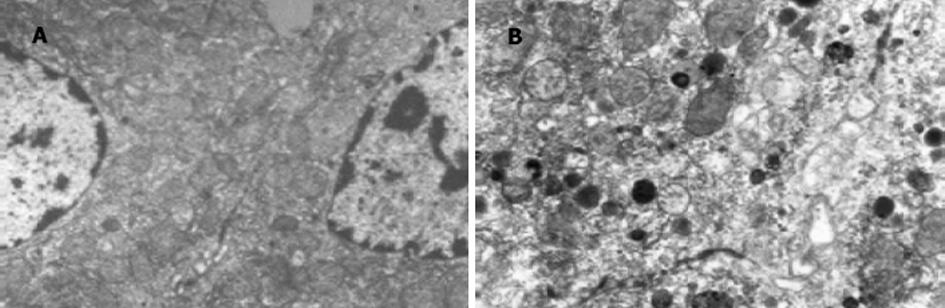
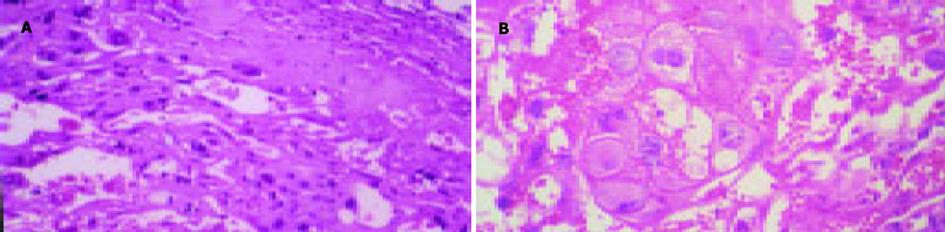
结果: 胆淤组和对照组比较, 孕鼠血清丙氨酸转氨酶(ALT)、门冬氨酸转氨酶(AST)、总胆汁酸(TBA)的差异有极显著性(AST: 3 784±155 vs 747±158; ALT: 7 076±220 vs 847±198; TBA: 78.5±4.5 vs 25.2±3.7; P均<0.01).胆淤组孕鼠肝脏光镜下见部分肝细胞有颗粒样变性和空泡变性, 电镜下见肝组织中毛细胆管扩张, 毛细胆管及肝细胞内见高电子致密物沉积.胆淤组孕鼠胎盘光镜下见部分滋养细胞颗粒样变性和空泡变性.
结论: 孕鼠肝内胆汁淤积症时高胆汁酸血症对孕鼠肝细胞和胎盘滋养细胞均有明显的细胞毒性作用.
引文著录: 邵勇, 姚珍薇, 李红霞, 丁敏, 吴味辛, 汪克健, 廖晓刚, 易永芬. 妊娠肝内胆汁淤积症对孕鼠肝脏和胎盘的毒性作用. 世界华人消化杂志 2005; 13(12): 1404-1407
Revised: April 1, 2005
Accepted: April 8, 2005
Published online: June 28, 2005
AIM: To investigate the toxic effect of intrahepatic cholestasis of pregnancy (ICP) on the liver and placenta in pregnant rats.
METHODS: The animal model of ICP was induced by 17-a-ethinylestradiol and progesterone. Pathohistological changes of the liver and placenta of the pregnant rats were observed under light and electronic microscope.
RESULTS: The levels of serum alanine transaminase (ALT), aspartate transaminase (AST), and total bile acid (TBA) were significantly higher in ICP rats than the control (AST: 3 784±155 vs 747±158; ALT: 7 076±220 vs 847±198; TBA: 78.5±4.5 vs 25.2±3.7; P<0.01 for all of the three). Granule- and vacuole-like cells were observed in liver of ICP rats under light microscope and Bile canaliculi were dilated and deposition of substances with high electronic density was found in bile canaliculi and hepatocytes of ICP rats by electronic microscopy. Granule- and vacuole-like changes were also found in some placenta syncytiotrophoblast of ICP rats by light microscopy.
CONCLUSION: ICP with hyperbileacidemia has marked toxicity to hepatocytes and placenta trophoblasts of pregnant rats.
- Citation: Shao Y, Yao ZW, Li HX, Ding M, Wu WX, Wang KJ, Liao XG, Yi YF. Toxic effect of pregnancy intrahepatic cholestasis on liver and placenta in pregnant rats. Shijie Huaren Xiaohua Zazhi 2005; 13(12): 1404-1407
- URL: https://www.wjgnet.com/1009-3079/full/v13/i12/1404.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v13.i12.1404
妊娠肝内胆汁淤积症(intrahepatic cholestasis of pregnancy, ICP)是妊娠期特有的并发症, 临床表现为妊娠中晚期出现皮肤瘙痒和/或黄疸, 肝功能异常, 孕妇血液中胆汁酸明显升高, 并可经胎盘转运至胎儿.大量临床研究显示, ICP孕妇血液中胆汁酸水平愈高, 胎儿窘迫发生率亦愈高[1].毒理学实验表明, 胆汁酸对动物细胞如肝细胞、内皮细胞、心肌细胞等具有浓度及时间依赖性细胞毒性作用[2-7].目前, ICP的病因仍然不甚清楚, 可能与机体雌孕激素代谢异常或肝脏对雌孕激素异常敏感有关[8-9]; ICP时容易发生胎儿窘迫和胎儿猝死, 其发生机制还不清楚, 没有有效的防治措施.我们应用雌孕激素建立ICP孕鼠模型, 观察孕鼠肝内胆汁淤积症高浓度胆汁酸对母体肝脏, 以及胎盘的影响及其意义.
所有动物由第三军医大学大坪医院实验动物中心提供.选择清洁级SD雌性大鼠20只, 体质量200-250 g, 鼠龄150-180 d, 在室温18-25℃、相对湿度60-70%的屏障系统内饲养, 不控制饲料和饮水.在发情期与雄性大鼠按4∶1同笼饲养, 每天观察阴栓脱落情况, 阴栓脱落日定为妊娠1 d, 一直饲养到妊娠15 d待用, 妊娠后的大鼠每隔3 d称体质量1次.
20只SD孕鼠随机分为2组, 每组10只.对照组: 自孕15 d起至孕19 d, 每天后肢内侧sc注射精制植物油2.5 mL/kg/d.胆淤模型组: 自孕15 d起至孕19 d, 每天后肢内侧sc注射孕酮(Sigma公司, 溶解于精制植物油)75 mg/kg穌和17-a-乙炔雌二醇(Sigma公司, 溶解于精制植物油)1.25 mg/(kg/d).两组孕鼠均于妊娠15 d、妊娠第21 d眶静脉采血1 mL, 采用日本Olympus Au400全自动生化分析仪测定血生化指标.孕鼠均于妊娠21 d断头处死, 取孕鼠肝脏、胎盘进行HE染色组织学检查, Olympus光学显微镜下观察拍照.肝脏组织学检查见小叶间胆管内有胆汁淤积, 部分肝细胞有空泡样变性, 但无肝细胞坏死, 血清总胆汁酸、转氨酶升高为模型成功.模型成功后, 于妊娠第21 d取孕鼠肝脏组织, 切取数块大小约1 mm3组织块, 快速放入25 g/L戊二醛-多聚甲醛固定液中, 4℃固定2 h以上, 经磷酸缓冲液浸洗后, 用10 g/L的四氧化锇后固定, 乙醇系列脱水, Epon812包埋, LKB超薄切片机切片, 铅、铀染色, HITACHI-600透射电镜观察拍照.
统计学处理 所得数据输入SPSS for Windows10.0统计软件包进行统计.
建立胆淤动物模型前胆淤组和对照组比较, ALT、AST和TBA的差异无显著性(P>0.05, 表1).建立胆淤动物模型后两组比较, ALT、AST和TBA的差异有极显著性(P<0.01, 表1).
对照组孕鼠肝脏外观表现为边缘锐利, 色泽红润; 胆淤模型组肝脏边缘钝厚, 色泽灰暗.光镜下对照组孕鼠肝细胞形态正常, 肝小叶结构正常; 胆淤模型组部分肝细胞有颗粒变性和空泡变性, 肝小叶结构正常(图1).电镜下胆淤模型组孕鼠肝组织中毛细胆管扩张, 毛细胆管及肝细胞内见高电子致密物沉积, 部分线粒体水肿(图2).
对照组孕鼠胎盘外观色泽红润; 胆淤模型组孕鼠胎盘色泽灰暗.光镜下对照组滋养细胞形态正常, 胎盘结构正常; 胆淤模型组部分滋养细胞颗粒变性和空泡变性, 合体滋养细胞变性更为明显, 绒毛间隙内胆汁淤积(图3).
尽管ICP的发病原因至今仍然不甚清楚, 但是许多研究表明, ICP的发生与妊娠时血液中雌孕激素水平增高或对雌孕激素过度敏感有关[8-14].有学者单纯使用雌激素[EE, 2.5 mg/(kg/d)]处理孕鼠诱导类似人ICP的大鼠模型, 引起孕鼠血循环中胆汁酸水平明显升高, 认为可能系因EE引起肝细胞器的某些功能受损, 胆汁分泌功能障碍, 肝细胞合成的胆汁酸不能正常引流到胆管系统, 而聚积在肝细胞及毛细胆管内而返流入血; 但是, 该模型显示肝脏在光镜下有少量点状坏死, 与人ICP时肝脏无点状坏死不符合[15].许多研究显示, 孕激素也参与了ICP的发病过程.孕激素可增加胆管树的通透性, 使胆汁酸等有机阴离子返流增加; 降低胆管膜Na+-K+-ATP酶活性, 减少Na+-K+-ATP酶依赖的胆盐转运; 引起细胞膜流动性下降, 阻碍载体移动; 引起肝细胞膜脂质成分异常, 影响胆汁分泌或使胆汁回流增加, 产生肝内胆汁淤积症的病理生理改变.因此, 我们联合应用雌激素[EE, 1.25 mg/(kg/d)]和孕激素[P, 75 mg/(kg/d)]建立ICP动物模型, 结果显示, 胆淤模型组孕鼠血液中ALT, AST和TBA水平显著高于对照组(P<0.01); 胆淤模型组孕鼠肝细胞出现颗粒样变性和空泡变性, 胆汁酸沉积, 电镜显示肝组织中毛细胆管扩张, 毛细胆管及肝细胞内见高电子致密物沉积, 部分线粒体水肿; 而胎盘绒毛肿胀, 间隙狭窄, 胆汁酸沉积, 合体滋养细胞空泡变性、水肿.这些变化与人ICP时生化特征和病理改变非常相似.有研究发现, 雌激素可促进细胞增殖, 抑制细胞凋亡, 对组织损伤具有保护作用[16-17]; 孕激素可调节雌激素促细胞增殖作用, 抑制细胞的过度增生[16].本研究应用低剂量的雌、孕激素建立孕鼠ICP模型, 雌、孕激素本身对孕鼠并无直接毒性作用.ICP时出现高胆汁酸血症是其重要特征, 血清胆汁酸水平升高是诊断ICP的可靠指标[1].本研究显示, 胆淤模型组孕鼠血液中总胆汁酸(TBA)水平显著高于对照组(P<0.01).
胆汁酸是一类既含有亲水基团(羟基、羧基和磺酰基), 又含有疏水基团(甲基、羟核脂酰侧链)的特殊物质.在空间配位上, 两类不同性质的基团恰好分别排在环戊烷多氢菲的两侧, 亲水基团均为a型, 甲基为b型, 故胆汁酸的主体构象具有亲水和疏水两个侧面, 这样的结构使胆汁酸表现出很强的界面活性, 从而在脂水两相间能够降低表面张力.正常情况下, 在肝细胞内形成的胆汁酸有胆酸和鹅脱氧胆酸分别与甘氨酸、牛磺酸结合, 形成甘氨胆酸、甘氨鹅脱氧胆酸、牛磺胆酸和牛磺鹅脱氧胆酸, 称为初级胆汁酸, 也称结合胆汁酸, 经肠道细菌作用后形成脱氧胆酸和石胆酸, 称为次级胆汁酸.胆盐通过窦状隙基膜上的特殊转运蛋白进入细胞内, 以囊泡形式跨越细胞, 从而由肝细胞胆管膜泌入胆汁, 当胆汁流损害引起胆汁淤积, 胆盐进入胆汁受阻, 导致胆盐在肝细胞内的聚集.胆汁淤积时胆盐在肝细胞内聚集被认为是肝脏损害的一个重要因素[18-25].在胆汁淤积患者血中以初级胆汁酸增加为主.鹅脱氧胆酸是初级胆汁酸, 对肝细胞具有明显细胞毒作用[26], 95%以上呈结合状态, 与甘氨胆酸和牛磺胆酸结合的比率为3∶2.在毒性胆盐中, 甘氨酸结合的胆盐比牛磺酸结合的毒性要大[27-28].另外, 在胆汁淤积时, 肝组织内鹅脱氧胆酸的浓度比其他毒性胆盐如脱氧胆酸和石胆酸要高[28], 这说明鹅脱氧胆酸的细胞毒性具有临床重要性.本研究显示, 胆淤模型组孕鼠肝细胞和胎盘滋养细胞均表现变性、水肿等细胞毒性改变.
ICP时, 由于孕妇高胆汁酸血症, 胆汁酸盐沉积于胎盘, 胎盘滋养细胞出现细胞毒性改变, 胎儿体内产生的代谢产物如胆汁酸通过胎盘转运到母体的功能受到影响, 而且母体血中呈高浓度胆汁酸状态, 使得胆汁酸由母体侧进入胎儿体内, 胎儿血液中的胆汁酸水平异常聚集升高, 胆汁酸可以通过被动扩散和钠依赖载体主动转运进入胎儿细胞内[29-30], 可能引起胎儿细胞毒性改变.大量临床研究发现, ICP孕妇血液中胆汁酸水平愈高, 胎儿窘迫发生率亦愈高.由此可见, 胆汁酸对胎儿的不良影响是明显的.但是, ICP时高胆汁酸血症对胎儿有何毒性作用, 是否为胎儿猝死的原因, 其作用机制是什么, 目前国内外文献尚无相关的研究报道.本研究建立的孕鼠肝内胆汁淤积症模型为进一步研究ICP对母体和胎儿的毒性作用提供了有利的工具.深入的研究很有必要, 可能为探讨ICP对母儿影响机制的研究寻找到一条新的研究方向.
编辑: 潘伯荣 审读: 张海宁
| 1. | Glantz A, Marschall HU, Mattsson LA. Intrahepatic cholestasis of pregnancy: Relationships between bile acid levels and fetal complication rates. Hepatology. 2004;40:467-474. [PubMed] [DOI] |
| 2. | Garner CM, Mills CO, Elias E, Neuberger JM. The effect of bile salts on human vascular endothelial cells. Biochim Biophys Acta. 1991;1091:41-45. [PubMed] [DOI] |
| 3. | Gumpricht E, Dahl R, Devereaux MW, Sokol RJ. Licorice compounds glycyrrhizin and 18beta-glycyrrhetinic acid are potent modulators of bile acid-induced cytotoxicity in rat hepatocytes. J Biol Chem. 2005;280:10556-10563. [PubMed] [DOI] |
| 4. | Rolo AP, Palmeira CM, Holy JM, Wallace KB. Role of mitochondrial dysfunction in combined bile acid-induced cytotoxicity: the switch between apoptosis and necrosis. Toxicol Sci. 2004;79:196-204. [PubMed] [DOI] |
| 5. | Gumpricht E, Dahl R, Devereaux MW, Sokol RJ. Beta-carotene prevents bile acid-induced cytotoxicity in the rat hepatocyte: Evidence for an antioxidant and anti-apoptotic role of beta-carotene in vitro. Pediatr Res. 2004;55:814-821. [PubMed] [DOI] |
| 6. | Wu Z, Lu Y, Wang B, Liu C, Wang ZR. Effects of bile acids on proliferation and ultrastructural alteration of pancreatic cancer cell lines. World J Gastroenterol. 2003;9:2759-2763. [PubMed] [DOI] |
| 7. | Araki Y, Andoh A, Bamba H, Yoshikawa K, Doi H, Komai Y, Higuchi A, Fujiyama Y. The cytotoxicity of hydrophobic bile acids is ameliorated by more hydrophilic bile acids in intestinal cell lines IEC-6 and Caco-2. Oncol Rep. 2003;10:1931-1936. [PubMed] [DOI] |
| 8. | Reyes H, Sjovall J. Bile acids and progesterone metabolites in intrahepatic cholestasis of pregnancy. Ann Med. 2000;32:94-106. [PubMed] [DOI] |
| 9. | Leslie KK, Reznikov L, Simon FR, Fennessey PV, Reyes H, Ribalta J. Estrogens in intrahepatic cholestasis of pregnancy. Obstet Gynecol. 2000;95:372-376. [PubMed] [DOI] |
| 10. | Rodriguez-Garay EA. Cholestasis: human disease and experimental animal models. Ann Hepatol. 2003;2:150-158. [PubMed] |
| 11. | Saint-marc Girardin MF. Hepatic complications of oral contraceptives. Contracept Fertil Sex (Paris). 1984;12:13-16. [PubMed] |
| 12. | Germain AM, Carvajal JA, Glasinovic JC, Kato CS, Williamson C. Intrahepatic cholestasis of pregnancy: an intriguing pregnancy-specific disorder. J Soc Gynecol Investig. 2002;9:10-14. [PubMed] [DOI] |
| 13. | Shi Q, Liu S, Xiong Q. The changes of serum estrogen, progesterone and the function of immune system in intrahepatic cholestasis of pregnancy. Zhonghua Fuchanke Zazhi. 1998;33:724-726. [PubMed] |
| 14. | Reyes H, Simon FR. Intrahepatic cholestasis of pregnancy: an estrogen-related disease. Semin Liver Dis. 1993;13:289-301. [PubMed] [DOI] |
| 16. | Wiebe JP, Lewis MJ, Cialacu V, Pawlak KJ, Zhang G. The role of progesterone metabolites in breast cancer: Potential for new diagnostics and therapeutics. J Steroid Biochem Mol Biol. 2005;93:201-208. [PubMed] [DOI] |
| 17. | Queiroga FL, Perez-Alenza MD, Silvan G, Pena L, Lopes C, Illera JC. Role of steroid hormones and prolactin in canine mammary cancer. J Steroid Biochem Mol Biol. 2005;94:181-187. [PubMed] [DOI] |
| 18. | Fickert P, Fuchsbichler A, Wagner M, Zollner G, Kaser A, Tilg H, Krause R, Lammert F, Langner C, Zatloukal K. Regurgitation of bile acids from leaky bile ducts causes sclerosing cholangitis in Mdr2 (Abcb4)knockout mice. Gastroenterology. 2004;127:261-274. [PubMed] [DOI] |
| 19. | Mohamed T, Oikawa S, Iwasaki Y, Mizunuma Y, Takehana K, Endoh D, Kurosawa T, Sato H. Metabolic profiles and bile acid extraction rate in the liver of cows with fasting-induced hepatic lipidosis. J Vet Med A Physiol Pathol Clin Med. 2004;51:113-118. [PubMed] [DOI] |
| 20. | Paszt A, Takacs T, Rakonczay Z, Kaszaki J, Wolfard A, Tiszlavicz L, Lazar G, Duda E, Szentpali K, Czako L. The role of the glucocorticoid-dependent mechanism in the progression of sodium taurocholate-induced acute pancreatitis in the rat. Pancreas. 2004;29:75-82. [PubMed] [DOI] |
| 21. | Edremitlioglu M, Oner G. The role of cholesterol on the pressure sensing ability of kidneys in rats. J Basic Clin Physiol Pharmacol. 2003;14:345-358. [PubMed] [DOI] |
| 22. | Fischer S, Muller I, Zundt BZ, Jungst C, Meyer G, Jungst D. Ursodeoxycholic acid decreases viscosity and sedimentable fractions of gallbladder bile in patients with cholesterol gallstones. Eur J Gastroenterol Hepatol. 2004;16:305-311. [PubMed] [DOI] |
| 23. | Marcil M, O'Connell B, Krimbou L, Genest J Jr. High-density lipoproteins: multifunctional vanguards of the cardiovascular system. Expert Rev Cardiovasc Ther. 2004;2:417-430. [PubMed] [DOI] |
| 24. | Poupon R. Molecular mechanisms of bile formation and cholestatic diseases. Bull Acad Natl Med. 2003;187:1261-1274. [PubMed] |
| 25. | Riegler FM, Consentini EP. Bile salts affect epithelial restitution. J Am Coll Surg. 2004;198:855-856. [PubMed] [DOI] |
| 26. | Miyazaki K, Nakayama F, Koga A. Effect of chenodeoxycholic and ursodeoxychlic acids on isolated adult human hepatocytes. Dig Dis Sci. 1984;12:1123-1130. [PubMed] [DOI] |
| 27. | Kimura T. Cytotoxicity of bile acids on cultured cells (author's transl). Nippon Shokakibyo Gakkai Zasshi. 1980;77:185-194. [PubMed] |
| 28. | Crosignani A, Podda M, Battezzati PM, Bertdini E, Zuim M, Watson D, Setchell KD. Changes in bile acid composition in patients with primary biliary cirrhosis induced by ursodeoxycholic acid administration. Hepatology. 1991;14:1000-1007. [PubMed] [DOI] |
| 29. | Donovan JM, Jackson AA. Transbilayer movement of fully ionized taurine-conjugated bile salts depends upon bile salt concentration, hydrophobicity, and membrane cholesterol content. Biochemistry. 1997;36:11444-11451. [PubMed] [DOI] |