修回日期: 2005-03-27
接受日期: 2005-04-01
在线出版日期: 2005-06-15
目的: 探讨鼠CD40配体基因体内抗肿瘤作用.
方法: 转染和未转染质粒pcDNA3.1+-mCD40L的H22细胞(1×106)分别种植到同源Balb/c小鼠左侧腹皮下.荷瘤小鼠(瘤结节最大直径约10 mm)瘤结节内直接注射pcDNA3.1+-mCD40L/脂质体复合物(治疗组)或者pcDNA3.1+、脂质体、培养基(对照组).每周测量各组小鼠皮下肿瘤结节的平均直径的大小, 绘制肿瘤生长曲线.RT-PCR法检测治疗组肿瘤组织mCD40L mRNA的表达.常规组织HE染色观察治疗组肿瘤组织病理变化.
结果: 注射转染了质粒的H22细胞的Balb/c小鼠皮下一直未见瘤结节出现.治疗组荷瘤小鼠瘤结节生长受到明显的抑制, 而对照组小鼠瘤结节生长没有抑制.用RT-PCR法从pcDNA3.1+-mCD40L治疗组的小鼠肿瘤组织中扩增出783 bp大小片段产物.病理检查发现治疗组肿瘤组织中有明显淋巴细胞浸润、大量凋亡小体和片状坏死.
结论: 质粒pcDNA3.1+-mCD40L转染后使H22细胞致瘤性明显下降.直接瘤内注射pcDNA3.1+-mCD40L能使荷瘤Balb/c小鼠瘤结节生长明显抑制.质粒pcDNA3.1+-mCD40L治疗组肿瘤组织中有mCD40L mRNA的表达和明显的炎症反应以及肿瘤细胞凋亡、坏死明显增加.
引文著录: 蒋永芳, 何艳, 张永红, 许允, 龚国忠. CD40配体基因对实验性肝细胞癌的治疗作用. 世界华人消化杂志 2005; 13(11): 1287-1290
Revised: March 27, 2005
Accepted: April 1, 2005
Published online: June 15, 2005
AIM: To investigate the anti-tumor effect of murine CD40 ligand gene in vivo.
METHODS: Parental H22 cells and H22 cells transfected with pcDNA3.1+-mCD40L (H22-CD40L)(1×106) were inoculated subcutaneously into the left flanks of syngenic Balb/c mice respectively. Tumor-bearing mice (tumor nodules were 10 mm in maximal diameter) were treated by intratumoral injection of either pcDNA3.1+-mCD40L/Transfectam (treating group) or Transfectam or pcDNA3.1+ or RPMI1640 (control). All the mice were monitored for tumor growth weekly. The mCD40L mRNA expression was detected by reverse transcription polymerase chain reaction (RT-PCR) and the histological changes were observed after routine HE staining.
RESULTS: All the mice inoculated with parental H22 cells developed a subcutaneous tumor, and the tumor size increased progressively within 3 weeks. However, the mice received H22-CD40L cells exhibited complete regression 2 weeks after inoculation. Tumor-bearing animals received Transfectam or pcDNA3.1+ or RPMI1640 had progressive tumor growth, while those treated with pcDNA3.1+-mCD40L exhibited a significant inhibition of tumor growth. A fragment of 783 bp corresponding to the mCD40L mRNA was amplified only from pcDNA3.1+-mCD40L treatmented tumors. Tumor samples from pcDNA3.1+-mCD40L-treated mice showed significant lymphocyte infiltration, apoptosis and confluent necrosis.
CONCLUSION: The tumorigenicity of CD40L-expressing cells abrogated when they were implanted subcutaneously. In vivo gene therapy for established liver tumor nodules in mice by intratumor injection of pcDNA3.1+-mCD40L led to significant tumor inhibition. mCD40L mRNA is expressed in pcDNA3.1+-mCD40L treated tumors. Intratumoral injection of pcDNA3.1+-mCD40L induces a strong inflammatory, mainly lymphocyte infltration and necrosis of tumor cells.
- Citation: Jiang YF, He Y, Zhang YH, Xu Y, Gong GZ. Therapeutic effect of CD40 ligand gene on hepatocelluLar carcinoma in mice. Shijie Huaren Xiaohua Zazhi 2005; 13(11): 1287-1290
- URL: https://www.wjgnet.com/1009-3079/full/v13/i11/1287.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v13.i11.1287
CD40配体(CD40L)是肿瘤坏死因子家族中的一员, 在激活的T细胞等细胞表面均有表达.CD40L通过与抗原提呈细胞(APC)上的CD40分子结合相互作用, 而激活APC以及激发细胞免疫和体液免疫反应[1].所以CD40L-CD40相互作用在介导抗肿瘤免疫反应方面起至关重要的作用.我们利用脂质体介导鼠CD40配体(mCD40L)基因治疗鼠肝细胞癌, 观察其抑瘤效果.
包含具有编码鼠CD40配体 cDNA全部编码区的真核表达载体(pcDNA3.1+-mCD40L)是我们先前的实验构建[2-3]; 大肠杆菌JM109, 购于美国Promega公司; Balb/c小鼠肝癌细胞株H22细胞购自武汉大学典型物保存中心; Balb/c小鼠购于中南大学湘雅二医院动物室; RPMI1640培养基和G418购自美国Gibico公司; 脂质体转染剂 Lipofectamine PlusTM Reagent和Transfectam购自美国Invitrogen公司.
pcDNA3.1+-mCD40L转染到H22细胞以前的实验已有报道[2-3].Balb/c小鼠肝癌模型采用皮下移植模型, 便于观察肿瘤生长情况.将H22细胞扩增培养后, 用灭菌的PBS液调至5×108个/L.取Balb/c小鼠30只, 常规消毒后在每只鼠的左侧腹sc 1×106个癌细胞(0.2 mL).2 wk后小鼠肝癌模型制作成功, 于接种处长出直径约1 cm 癌肿.取雌性、4周龄Balb/c小鼠20只, 质量15-20 g, 普通饲料喂养, 自由进食.在动物实验室饲养1 wk适应环境后, 按随机分组的方法分为两组, 每组10只.实验组每只Balb/c小鼠左侧腹sc转染了质粒pcDNA3.1+-mCD40L的H22细胞1×106 个; 对照组每只Balb/c小鼠左侧腹sc未转染质粒pcDNA3.1+-mCD40L的H22细胞1×106 个.每天观察小鼠注射H22细胞处皮肤, 是否有瘤结节出现.每周用游标卡尺测量出现瘤结节小鼠皮下肿瘤的长径与宽径(mm), 取其直径平均值, 并绘制肿瘤生长曲线.当Balb/c小鼠肝癌模型的瘤结节长至平均直径约为1.0 cm, 随机分组后(每组10只), 治疗组每只小鼠瘤体内注入pcDNA3.1+-mCD40L质粒/阳离子脂质体复合物100 μL, 含10μL pcDNA3.1+-mCD40L质粒, 0.2 μL阳离子脂质体.对照组1每只小鼠瘤体内注入含10 μL质粒 pcDNA3.1+的无血清培养基100 μL.对照组2每只小鼠瘤体内注入含0.2 μL阳离子脂质体的无血清培养基100 μL.对照组3每只小鼠瘤体内注入无血清培养基100 μL.定期测量各组小鼠皮下肿瘤结节的平均直径的大小, 绘制肿瘤生长曲线.采用RT-PCR法检测肿瘤组织mCD40L的表达情况, 用TRIzol试剂抽提肿瘤组织RNA, RNA逆转录后在引物5'-GACGCTAGCATGATAGAAACATACAGCCAACCT-3'和5'-GCCGAATTCTCAGAGTTTGAGTAAGCCAAAAGA-3'作用下进PCR扩增.荷瘤小鼠瘤结节内注射质粒pcDNA3.1+-mCD40L后14 d, 处死小鼠, 提取肿瘤组织, 组织标本用40 g/L的甲醛固定, 石蜡包埋, 连续切片, 常规HE染色.
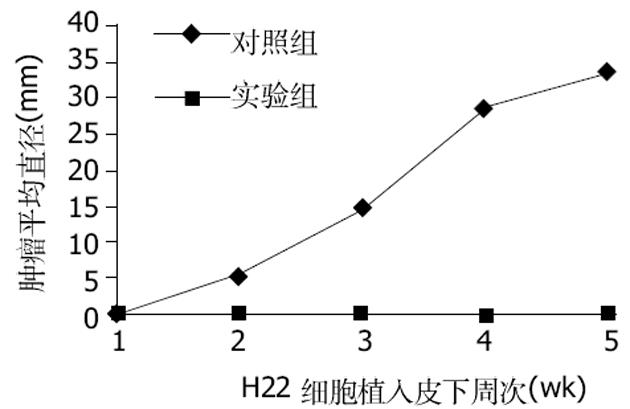
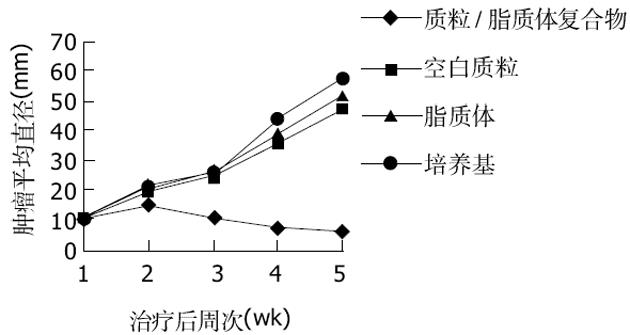
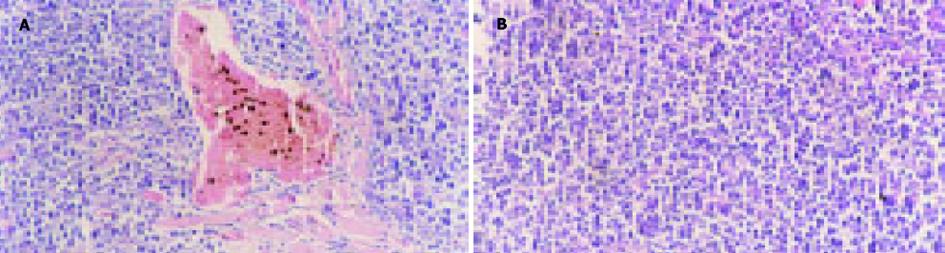
对照组(注射没有转染质粒的H22细胞)小鼠在7 d均长出了肉眼可见的瘤结节, 随着时间的推移, 对照组小鼠瘤结节逐渐增大.实验组(注射转染了质粒的H22细胞)小鼠一直未见瘤结节出现(图1).说明转染了质粒pcDNA3.1+-mCD40L后H22细胞在同源Balb/c小鼠体内成瘤性降低.治疗组小鼠瘤结节内注入质粒/阳离子脂质体复合物后, 肿瘤的生长明显抑制, 而各对照组肿瘤生长没有受到抑制(图2).说明mCD40L基因能有效抑制肿瘤的生长.采用RT-PCR法检测各组肿瘤组织mCD40LmRNA的表达, 结果发现治疗组的RT-PCR产物可见783 bp条带, 其余各组未见此条带.证明治疗组肿瘤组织中有mCD40LmRNA的表达.治疗组荷瘤小鼠瘤结节内注射质粒pcDNA3.1+-mCD40L后14 d, 病理检测发现肿瘤组织有多量的淋巴细胞浸润、大量的凋亡小体及片状坏死(图3A).而各对照组肿瘤组织未见明显淋巴细胞浸润和肿瘤细胞变性、坏死(图3B).说明瘤内注射质粒pcDNA3.1+-mCD40L能诱导肿瘤组织内淋巴细胞浸润, 增加肿瘤细胞凋亡和坏死.
CD40配体(CD40L)又称作CD154, 属于肿瘤坏死因子基因超家族中的一员, 在激活的T细胞等多种细胞表面均有CD40L的表达, 同样包括B细胞和多种肿瘤细胞在内细胞表面有CD40分子的表达, CD40L和CD40相互作用, 能激活B细胞和前凝血质, 促进黏附因子、细胞因子、化学因子和MMP的释放[1].其实质是诱发体液免疫和细胞介导的免疫反应.如Ritchie et al[4]证实CD40L激活B细胞通过直接或者间接激活CD8+T细胞诱导抗原特异性抗肿瘤免疫反应, 能明显抑制肿瘤细胞的生长.正由于CD40L/CD40相互作用所具有的多种生物学活性, 将外源目的基因CD40L基因导入机体内, 可能产生强烈的抗肿瘤免疫反应, 已有不少学者开展了对如肺癌[5-6]、结肠癌[7]、泌尿系肿瘤[8-10]、胸膜间皮瘤[11]、胃癌[12]、黑色素瘤[13]以及淋巴瘤[14-15]、急性白血病[16]和慢性B淋巴细胞性白血病[17]等方面的研究, 结果表明鼠CD40L基因均能激活T细胞、上调IL-12等细胞因子水平和增加NK细胞活力等保护性免疫反应和增加癌细胞凋亡, 从而使荷瘤鼠的瘤结节退化, 能持久抑制再次注入的癌细胞的生长, 动物存活期延长, 以及能诱导明显的抗白血病免疫反应.用CD40L转染DC细胞后能促进DC细胞成熟和分泌IL-12[18-19], 所以通过转染CD40L基因制备DC瘤苗同样可以治疗肿瘤, 如Sato et al[20]联合DC细胞和用CD40L激活后的自体T细胞 以及Hao et al[21]用聚乙烯二乙醇将DC细胞和有CD40L表达的骨髓瘤细胞J558融合, 形成一种新颖的融合杂合瘤苗, 能诱导更强的CD4+和CD8+T细胞介导的CTL反应和保护性抗肿瘤细胞免疫.杂合瘤苗可能成为一种有吸引力的免疫治疗癌症策略.总之, CD40L基因的治疗为癌症的基因治疗开辟一条新的途径.
Schmitz et al[22]首先利用腺病毒介导鼠CD40L基因治疗鼠原位肝细胞癌, 能使荷瘤大鼠瘤结节完全消退; 经过治疗后70%的荷瘤大鼠能长时间存活; 组织病理检查发现肿瘤组织中有大量淋巴细胞浸润和肿瘤细胞凋亡增加; 其机制是诱导CD8+T细胞介导的免疫反应、增加血清IL-12水平和增强NK细胞活力.Afford et al[23]认为该研究利用腺病毒和CD40配体基因为肝癌的基因治疗开辟了一条新的途径, 同时认为CD40L基因联合其他细胞因子治疗肝癌可能疗效更好.Yanagi et al[24]用腺病毒介导CD40配体基因转染肝癌细胞, 能使转染后的肝癌细胞致瘤性下降, 同样认为CD40L基因联合其他免疫佐剂可能发挥更强的抗肿瘤免疫反应.
我们用带有CD40L基因的质粒pcDNA3.1+-mCD40L转染H22细胞后, H22细胞在同源小鼠中的致瘤性明显下降; 用脂质体介导pcDNA3.1+-mCD40L直接在荷瘤小鼠瘤结节内注射, 能使荷瘤小鼠瘤结节生长明显抑制; 组织病理学检查发现肿瘤组织中有明显的淋巴细胞浸润、大量凋亡小体以及片状坏死; 与Schmitz et al[22]和Yanagi et al[24]的研究一致.RT-PCR法检测肿瘤组织中有mCD40LmRNA的表达, 说明瘤内注射质粒pcDNA3.1+-mCD40L能诱导肿瘤组织内淋巴细胞浸润, 增加肿瘤细胞凋亡和坏死, 从而抑制瘤结节生长.我们观察到了CD40L基因对实验性肝细胞癌有一定的治疗作用, 但其具体的作用机制、毒副作用有待进一步研究.
编辑: 潘伯荣 审读: 张海宁
| 1. | Schonbeck U, Mach F, Libby P. CD154 (CD40 ligand). Int J Biochem Cell Biol. 2000;32:687-693. [PubMed] [DOI] |
| 3. | Jiang YF, He Y, Gong GZ, Chen J, Yang CY, Xu Y. Construction of recombinant eukaryotic expression palsmid containing murine CD40 ligang gene and its expression in H22 cells. World J Gastroenterol. 2005;11:182-186. [PubMed] [DOI] |
| 4. | Ritchie DS, Yang J, Hermans IF, Ronchese F. B-Lymphocytes activated by CD40 ligand induce an antigen-specific anti-tumour immune response by direct and indirect activation of CD8 (+) T-cells. Scand J Immunol. 2004;60:543-651. [PubMed] [DOI] |
| 5. | Masahiro N, Kazuyoshi I, Tsutomu K, Wakayama H, Horio Y, Sekido Y, Hara T, Hashimoto N, Takahashi M, Shimokata K. Induction of antitumor immunity by transduction of CD40 ligand gene and interferon-gamma gene into lung cancer. Cancer Gene Ther. 2001;8:421-429. [PubMed] [DOI] |
| 6. | Tada Y, O-Wang J, Yu L, Shimozato O, Wang YQ, Takiquchi Y, Tatsumi K, Kuriyama T, Takemaga K, Sakiyama S. T-cell-dependent antitumor effects produced by CD40 ligand expressed on mouse lung carcinoma cells are linked with the maturation of dendritic cells and secretion of a variety of cytokines. Cancer Gene Ther. 2003;10:451-456. [PubMed] [DOI] |
| 7. | Buning C, Kruger K, Sieber T, Schoeler D, Schriever F. Increased expression of CD40 ligand on activated T cells of patients with colon cancer. Clin Cancer Res. 2002;8:1147-1151. [PubMed] |
| 8. | Loskog A, Totterman TH, Bohle A, Brandau S. In vitro activation of cancer patient-derived dendritic cells by tumor cells genetically modified to express CD154. Cancer Gene Ther. 2002;9:846-853. [PubMed] [DOI] |
| 9. | Loskog A, Bjorkland A, Brown MP, Korsgren O, Malmstrom PU, Totterman TH. Potent antitumor effects of CD154 transduced tumor cells in experimental bladder cancer. J Urol. 2001;166:1093-1097. [PubMed] [DOI] |
| 10. | Hussain SA, Ganesan R, Hiller L, Murray PG, el-Magraby MM, Young L, James ND. Proapoptotic genes BAX and CD40L are predictors of survival in transitional cell carcinoma of the bladder. Br J Cancer. 2003;88:586-592. [PubMed] [DOI] |
| 11. | Friedlander PL, Delaune CL, Abadie JM, Touos M, LaCour J, Marrero L, Zhong Q, Kolls JK. Efficacy of CD40 ligand gene therapy in malignant mesothelioma. Am J Respir Cell Mol Biol. 2003;29:321-330. [PubMed] [DOI] |
| 12. | Yamaguchi H, Tanaka F, Sadanaga N, Ohta M, Tnoue H, Mori M. Stimulation of CD40 inhibits Fas- or chemotherapy-mediated apoptosis and increases cell motility in human gastric carcinoma cells. Int J Oncol. 2003;23:1697-1702. [PubMed] [DOI] |
| 13. | Peter I, Nawrath M, Kamarashev J, Odermatt B, Mezzacasa A, Hemini S. Immunotherapy for murine K1735 melanoma: combinatorial use of recombinant adenovirus expressing CD40L and other immunomodulators. Cancer Gene Ther. 2002;9:597-605. [PubMed] [DOI] |
| 14. | Meziane el K, Bhattacharyya T, Armstrong AC, Qian C, Hawkins RE, Stern PL, Dermime S. Use of adenoviruses encoding CD40L or IL-2 against B cell lymphoma. Int J Cancer. 2004;111:910-920. [PubMed] [DOI] |
| 15. | Jin Z, Teramoto N, Hayashi K, Liu YX, Jin G, Oka T, Takahashi K, Yoshino T, Akagi T. CD40 ligand stimulation inhibits the proliferation of mantle cell lymphoma lines. Anticancer Res. 2004;24:691-697. [PubMed] |
| 16. | Saudemont A, Buffenoir G, Denys A, Desreumaux P, Jouy N, Hetuin D, Bauters F, Fenanx P, Quesnel B. Gene transfer of CD154 and IL12 cDNA induces an anti-leukemic immunity in a murine model of acute leukemia. Leukemia. 2002;16:1637-1644. [PubMed] [DOI] |
| 17. | Wendtner CM, Kofler DM, Theiss HD, Kurzeder C, Buhmann R, Schweighofer C, Perabo L, Danhauser-Riedl S, Baumert J, Hiddemann W. Efficient gene transfer of CD40 ligand into primary B-CLL cells using recombinant adeno-associated virus (rAAV) vectors. Blood. 2002;100:1655-1661. [PubMed] |
| 18. | Machey MF, Wang Z, Eichelberg K, Germain RN. Distinct contributions of different CD40 TRAF binding sites to CD154-induced dendritic cell maturation and IL-12 secretion. Eur J Immunol. 2003;33:779-789. [PubMed] [DOI] |
| 19. | Onaitis MW, Kalady MF, Emanis S, Abdel-Wahab Z, Tyler DS, Pruill SK. CD40 ligand is essential for generation of specific cytotoxic T cell responses in RNA-pulsed dendritic cell immunotherapy. Surgery. 2003;134:300-305. [PubMed] [DOI] |
| 20. | Sato T, Terai M, Yasuda R, Watanabe R, Berd D, Mastrangelo MJ, Hasumi K. Combination of monocyte-derived dendritic cells and activated T cells which express CD40 ligand: a new approach to cancer immunotherapy. Cancer Immunol Immunother. 2004;53:53-61. [PubMed] [DOI] |
| 21. | Hao S, Bi X, Xu S, Wei Y, Wu X, Guo X, Carlsen S, Xiang J. Enhanced antitumor immunity derived from a novel vaccine of fusion hybrid between dendritic and engineered myeloma cells. Exp Oncol. 2004;26:300-306. [PubMed] |
| 22. | Schmitz V, Barajas M, Wang L, Peng D, Duarte M, Prieto J, Qian C. Adenovirus-mediated CD40 ligand gene therapy in a rat model of orthotopic hepatocellular carcinoma. Hepatology. 2001;34:72-81. [PubMed] [DOI] |
| 23. | Afford SC, Young LS. Gene therapy for hepatocellular carcinoma teaching old dogs new tricks. Hepatology. 2001;34:207-209. [PubMed] [DOI] |
| 24. | Yanagi K, Nagayama Y, Nakao K, Saeki A, Matsumoto K, Ichikawa T, Ishikawa H, Hamasaki K, Ishii N, Eguchi K. Immuno-gene therapy with adenoviruses expressing fms-like tyrosine kinase 3 ligand and CD40 ligand for mouse hepatoma cells in vivo. Int J Oncol. 2003;22:345-351. [PubMed] [DOI] |