修回日期: 2004-10-07
接受日期: 2004-10-20
在线出版日期: 2005-01-01
目的: 探讨选择性诱导型一氧化氮合酶(iNOS)抑制剂氨基胍(AG)在大鼠胰腺缺血/再灌注损伤(I/R)中的作用.
方法: 建立大鼠胰腺缺血/再灌注模型. I/R组胰腺缺血30 min, 再灌注时间分别为2、4、6、12、24 h; AG组静脉注射氨基胍(40、60、80 mg/kg); 对照组注射等量的生理盐水. 使用硝酸还原酶法和碘-淀粉比色法分别检测血清一氧化氮(NO)水平及淀粉酶活性随再灌注时间的变化, 定量分析结构型一氧化氮合酶(cNOS)和诱导型一氧化氮合酶(iNOS)在胰腺组织中的活性, 并对胰腺进行组织形态学检查和免疫组化分析.
结果: I/R组血清NO水平和淀粉酶活性明显升高, 再灌注4 h后NO水平达到高峰, 淀粉酶活性开始升高; AG组血清NO水平及淀粉酶活性低于I/R组(P<0.01). I/R组再灌注4 h后iNOS活性显著增强, AG组iNOS活性明显下降(P<0.01). 再灌注6 h后I/R组开始出现胰腺损伤的表现, AG组未见胰腺组织明显损害表现. 再灌注4 h后I/R组见iNOS强阳性染色, AG组中未见iNOS阳性染色, 二者比较有显性差异(439±127 vs 33±4, P<0.01).
结论: 选择性iNOS抑制剂氨基胍在大鼠胰腺缺血/再灌注中起到保护作用.
引文著录: 李柏峰, 刘永锋, 夏丽萍, 程颖, 成东华, 王晓东, 李铁民, 赵宁. iNOS抑制剂对大鼠胰腺缺血/再灌注损伤的保护作用. 世界华人消化杂志 2005; 13(1): 44-48
Revised: October 7, 2004
Accepted: October 20, 2004
Published online: January 1, 2005
AIM: To investigate the effect of inducible nitric oxide synthase (iNOS) inhibitor animoguanidine (AG) during ischemia/reperfusion (I/R) in rat pancreas.
METHODS: The models of sham-operation and pancreas ischemia were established in rats. The splenic artery of rats in I/R group was occluded reversibly by microsurgical clip for 30 minutes, and the animals were killed after 2, 4, 6, 12, or 24 hours of reperfusion. Aminoguanidine was given to the rats in AG group (40, 60, 80 mg/kg; iv). Rats in control group received saline only. The serum nitric oxide (NO) level and amylase activity were detetermined with nitrate reductase and iodine-starch chromatometry. Pancreas constitutive NOS (cNOS) and iNOS activities were measured using NOS Test Kit. Pancreas sections were evaluated by light microscopy after HE and immunohistochemistry staining.
RESULTS: In I/R group, serum NO level and amylase activity were increased significantly after 4-hour reperfusion. The NO level reached the peak and the amylase activity began to rise at 4 hour. After the administration of aminoguanidine (80 mg/kg), the NO level and amylase activity became much lower than those in the I/R group (P < 0.01). After 4-hour reperfusion, tissue iNOS activity was increased significantly in I/R group, but remained normal in AG group (P < 0.01). Six, twelve, and twenty-four hours after reperfusion, pancreas injury was observed in I/R group, but no siginificant changes occurred in pancreas after animoguanidine (80 mg/kg) administration. Four hours after reperfusion, the positive rate of iNOS in the specimens from I/R group was significantly higher than that in AG (80 mg/kg) group (439±127 vs 33±4, P < 0.01). The activity of cNOS showed no significant difference between any two groups.
CONCLUSION: Selective iNOS inhibitor aminoguanidine has protective effect against the ischemia/reperfusion injury in rat pancreas.
- Citation: Li BF, Liu YF, Xia LP, Cheng Y, Cheng DH, Wang XD, Li TM, Zhao N. Protcetive effect of iNOS inhibitor on pancreas ischemia/reperfusion injury in rats. Shijie Huaren Xiaohua Zazhi 2005; 13(1): 44-48
- URL: https://www.wjgnet.com/1009-3079/full/v13/i1/44.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v13.i1.44
缺血/再灌注损伤是胰腺移植术后并发急性胰腺炎的主要原因之一[1-2]. 再灌注期间, 氧自由基的产生是造成损伤的重要原因, 一氧化氮(NO)作为一种反应性极强的自由基, 具有非常强烈的细胞毒性作用; 但同时NO还有显著舒张血管的功能, 对于改善胰腺移植术后微循环有积极作用. 因此, 如何利用NO的保护作用并避免其损害, 有着重要的意义. 本实验建立大鼠胰腺缺血/再灌注模型, 探讨胰腺缺血/再灌注损伤过程中一氧化氮合酶(NOS)各种亚型的表达情况, 以及诱导型一氧化氮合酶(iNOS)抑制剂在胰腺缺血/再灌注损伤中起到怎样的作用.
♂Wistar大鼠, 质量250±20 g, 随机分为: 对照组(control), 仅游离胃脾韧带和腹腔动脉; 缺血/再灌注组(I/R), 夹闭脾动脉造成胰腺体尾部缺血[3], 30 min后开放, 恢复灌注之前, 经阴茎背静脉注入与实验组等量的生理盐水, 分别于再灌注后0, 2, 4, 6, 12, 24 h处死动物, 并迅速开腹取胰腺; 氨基胍处理组(AG), 缺血30 min后, 恢复灌注之前, 注入盐酸氨基胍溶液, 剂量分别为40, 60, 80 mg/kg, 再灌注4, 6 h后处死动物, 迅速开腹取胰腺. 将胰腺标本分为2部分, 一部分放入40 g/L的PBS甲醛溶液中固定, 另一部分深低温冰箱-70 ℃保存. 所采集的血液不经抗凝, 2000 r/min离心10 min, 取血清-20 ℃冻存.
血清中NO水平使用硝酸还原酶法, 特异性将硝酸盐还原为亚硝酸盐得以检测. 血清中的淀粉酶的活性使用碘-淀粉比色法检测. 胰腺组织中cNOS和iNOS活性使用NOS测定试剂盒分别检测, 以每克组织每分钟生成1 μmol NO为一个酶活力单位. 按照标准步骤将胰腺组织制成石蜡切片, 光学显微镜下病理形态学检查. 使用特异性抗iNOS的第一抗体, 以及SABC试剂盒对石蜡切片染色, 对胰腺组织进行免疫组织化学分析, 观察iNOS在胰腺组织种表达的部位.
统计学处理 连续数据以平均值±标准差(mean±SD)表示, 使用SPSS10.0软件系统对实验结果进行统计学分析, 各组间差异进行方差分析.
对照组血清中NO很低. I/R组再灌注4 h后, 血清NO水平明显升高, 达到高峰, 与对照组差异显著(P<0.01, 表1); 随着再灌注时间延长, NO水平降至正常. AG组中氨基胍剂量为80 mg/kg时, NO水平明显低于I/R-4 h组(P<0.01); 而与对照组无明显差异(P>0.05). 氨基胍剂量为40, 60 mg/kg时, 作用不明显(P>0.05, 表2).
I/R组血清淀粉酶均显著升高, 其中以再灌注4 h以后各组升高更为明显(表1). 统计学分析, 对照组和I/R-0 h组之间无差别(P>0.05); I/R-4, I/R-6, I/R-12, I/R-24 h各组之间无差异(P>0.05). 而(对照组和I/R-0 h组)与(I/R-4, I/R-6、I/R-12、I/R-24 h各组)两大组之间的组间差异具有统计学意义(P<0.01). AG组中氨基胍剂量为80 mg/kg时, 血清淀粉酶水平均明显低于对应非治疗组(P<0.01, 表2).
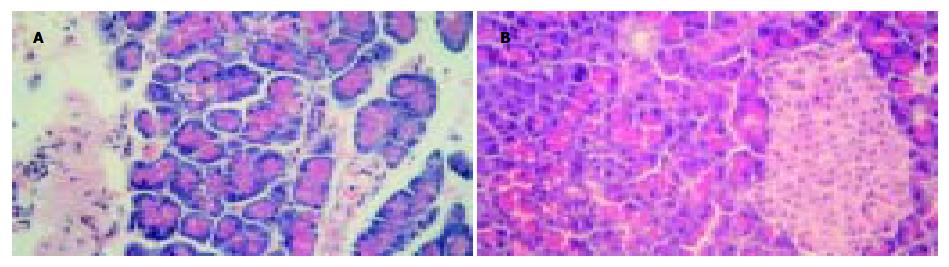
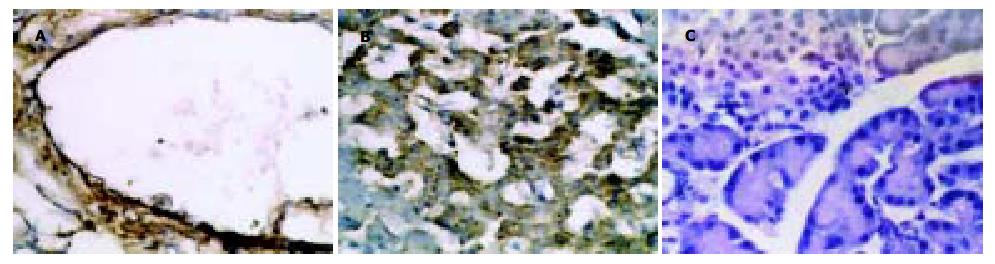
再灌注6 h后, I/R组开始出现胰腺损伤的表现, 如毛细血管扩张, 周围水肿, 静脉扩张并充血(图1A); 使用氨基胍后, 再灌注6 h未见胰腺组织明显损害表现(图1B). I/R组再灌注4 h后, iNOS活性显著增强, 高于对照组(P<0.01). AG组(80 mg)iNOS活性明显低于I/R-4 h组(P<0.01). 各组中cNOS均无变化(P>0.05, 表3). 再灌注4 h后, I/R组发现iNOS强阳性染色, iNOS的染色主要集中于血管内膜、平滑肌和胰岛细胞的胞质内(图2A-B); 使用氨基胍后, 组织中未见iNOS阳性染色(图2C).
一氧化氮(NO)在生物体内可转化成过氧化亚硝酸盐(﹒ONOOˉ˙), 是一种氧化性极强且性质非常稳定的自由基, 具有非常强烈的细胞毒性作用[4]; 在胰腺缺血/再灌注损伤中, NO是一种重要的炎症因子[5,6]. 同时NO还有舒张血管, 改善微循环灌流, 保护组织器官的作用[7]. iNOS可在内毒素和淋巴因子的刺激下迅速表达, 产生高浓度的NO, 所以有着非常重要的病理作用[8]. Takacs et al[9]给大鼠注射NOS底物L-精氨酸, 6 h后动物血清淀粉酶升高, 12 h后达到峰值, 同时发现胰腺血管通透性升高, 蛋白渗出, 以及组织损伤等急性胰腺炎的证据; 使用了NOS抑制剂L-NAME后, 虽然组织损伤未见明显改善, 但淀粉酶水平显著下降, 胰腺水肿减轻. 相应的研究还发现, 重症急性胰腺炎体内iNOS活性上调, 胰腺微循环中白细胞黏附的数量及出血坏死型胰腺炎的病理形态改变与胰腺组织内iNOS的表达和血中NO的水平正相关, 从而指出在胰腺炎的发展过程中iNOS和NO起着不利的作用[10-14]. Cuzzocrea et al[15]利用基因敲除技术建立iNOS基因缺陷的小鼠模型, 证实了iNOS的表达在急性胰腺炎中起到了重要的致炎作用. Zhou et al[16]综述了急性胰腺炎中对胰腺微循环有损害作用的影响因素, 指出NO同缓激肽、血小板活化因子、内皮素等一起促进了胰腺微循环衰竭的进展. Leindler et al[17]和Viola et al[3]在大鼠胰腺缺血/再灌注模型中发现, 胰腺缺血/再灌注损伤可导致严重的急性出血坏死性胰腺炎, 在这一过程中, 胰腺和肺组织中均有iNOS过度表达的趋势, 由此引起的NO过量产生介导了胰腺和肺组织的氧化损伤, 并与细胞凋亡密切相关. McDonald et al[18]在研究中发现, 钙蛋白酶抑制剂I能够减轻胰腺缺血/再灌注损伤引起的机体循环衰竭和多脏器功能不全, 其机制可能与防止了NF-κB和iNOS的表达有关. Duchen综合了以往的文献提出[19], 在组织器官缺血/再灌注损伤中, 细胞内钙超载与NO的大量产生有关, 他们共同参与了细胞内线粒体膜的破坏, 最终引起细胞死亡. Pi et al[20]和Wakai[21]发现, 在胰腺保存液中加入腺苷抑制物可以通过减少NO的增加, 从而降低髓过氧化物酶活性和脂过氧化物水平, 减轻了大鼠胰腺移植中的炎症反应. Yamamoto et al[22]建立了狗胰腺缺血/再灌注模型, 发现细胞因子IL-1β抑制物对缺血胰腺的保护作用, 当IL-1βmRNA表达下调时, 胰淀粉酶和胰脂肪酶水平也降低, 胰腺损伤减轻. 而IL-1β可诱导iNOS大量表达[23], 进而他们提出一些致炎细胞因子对胰腺组织的损害与iNOS的过度表达关系密切. 然而, Svensson et al[24]在应用非选择性NOS抑制剂后发现, 抑制NO的合成将造成大鼠本身胰腺和移植胰腺血流量减少; 而提供NOS底物虽然会导致大鼠胰腺形态学上的不良改变, 但可以改善胰腺组织血液灌流[25]. 其他一些研究者也曾得出过类似结论[26-29], 他们在大鼠的胰腺移植或胰腺缺血/再灌注模型中发现, 提供内源或外源NO可以改善胰腺微循环灌流, 减轻水肿和中性粒细胞的浸润, 从而对胰腺起到保护作用. 因此, NO在胰腺移植缺血/再灌注损伤中所起到的作用比较复杂, 尚未得到统一的认识. 经过比较这两类结论相反的研究, 我们发现后者所使用的均为非特异性的NOS抑制物[26,27], 而且结果多于胰腺再灌注120-240 min后得出[28-30]. 这说明在胰腺缺血/再灌注过程中, 动物体内NO水平可能存在变化, 因而在不同时段起着不同的作用, 而且NOS的各种亚型对胰腺缺血/再灌注损伤起到作用可能是不一样的. 那么, 随着胰腺恢复灌注时间的延长, 体内NOS和NO发生了什么变化, 对胰腺组织又有怎样的作用呢? 近年, 氨基胍作为iNOS的选择性抑制剂其特异性已得到广泛证实, 他对iNOS的抑制活性比对eNOS和nNOS分别强500倍和50倍, 而且急性毒性较低, 很有临床应用前景[11]. 在本实验中, 大鼠胰腺体尾部缺血后再灌注4 h, 内源性开始NO大量产生, 此时iNOS表达在胰腺组织上且活性远高于对照组, 同时发现了急性胰腺炎的证据; 而cNOS没有明显变化. 再灌注期使用选择性iNOS抑制剂氨基胍后, 这些变化都得到改善. 这说明胰腺缺血/再灌注损伤后, 胰腺炎的发生发展与iNOS关系密切. 基于以往的有关文献和本实验的结果可以得出这样的结论, 胰腺缺血/再灌注早期(0-2 h左右), NO主要由cNOS催化产生, 可以起到舒张胰腺血管, 改善微循环血流, 减轻组织水肿和炎症细胞浸润. 但随着再灌注早期的NO大量消耗, 这种保护作用减弱, 适当补充外源性NO或NOS的底物, 可能延续这种保护作用, 继续发挥舒张血管、缓解移植物血管痉挛的作用[27], 对于缺血/再灌注损伤造成的术后血栓形成及移植后胰腺炎的发生都有一定的预防作用. 而再灌注超过一定时间后(4 h以上), 一些在缺血/再灌注过程中大量表达的炎症递质如TNF-α L-1β等在分子水平上激活iNOS[23,31,32], 使其过量表达, 进一步引起NO浓度异常升高, 使后者作为自由基的细胞毒性充分表现出来, 并掩盖了他在改善微循环方面的保护作用. 因此iNOS和NO成为介导胰腺缺血/再灌注损伤的重要因素, 导致了移植后胰腺炎的发生发展. 使用选择性iNOS抑制剂, 虽然不能阻止炎症递质的产生, 但可以通过抑制iNOS的过量表达, 避免NO的异常增多, 在减少NO细胞毒性的同时, 保留其正常浓度下的有益作用, 减轻胰腺缺血/再灌注损伤, 在胰腺移植中起到保护作用.
编辑: 潘伯荣 审读: 张海宁
| 1. | Sweiry JH, Mann GE. Role of oxidative stress in the pathogenesis of acute pancreatitis. Scand J Gastroenterol Suppl. 1996;219:10-15. [PubMed] |
| 2. | Benz S, Bergt S, Obermaier R, Wiessner R, Pfeffer F, Schareck W, Hopt UT. Impairment of microcirculation in the early reperfusion period predicts the degree of graft pancreatitis in clinical pancreas transplantation. Transplantation. 2001;71:759-763. [PubMed] |
| 3. | Viola G, al-Mufti RA, Sohail M, Williamson RC, Mathie RT. Nitric oxide induction in a rat model of selective pancreatic ischemia and reperfusion. Hepatogastroenterology. 2000;47:1250-1255. [PubMed] |
| 4. | Butler AR, Flitney FW, Williams DL. NO, nitrosonium ions, nitroxide ions, nitrosothiols and iron-nitrosyls in biology: a chemist's perspective. Trends Pharmacol Sci. 1995;16:18-22. [PubMed] |
| 5. | Hotter G, Pi F, Sanz C, Peralta C, Prats N, Gelpi E, Badosa F, Fernández-Cruz L, Roselló-Catafau J. Endothelin mediated nitric oxide effects in ischemia--reperfusion associated with pancreas transplantation. Dig Dis Sci. 1998;43:2627-2633. [PubMed] |
| 6. | Peralta C, Hotter G, Closa D, Pi F, Badosa F, Gelpí E, Roselló-Catafau J. Nitric oxide enhances endothelin production in pancreas transplantation. Pancreas. 1997;14:369-372. [PubMed] |
| 7. | Konturek SJ, Szlachcic A, Dembinski A, Warzecha Z, Jaworek J, Stachura J. Nitric oxide in pancreatic secretion and hormone-induced pancreatitis in rats. Int J Pancreatol. 1994;15:19-28. [PubMed] |
| 8. | Mizutani A, Maki H, Torii Y, Hitomi K, Tsukagoshi N. Ascorbate-dependent enhancement of nitric oxide formation in activated macrophages. Nitric Oxide. 1998;2:235-241. [PubMed] |
| 9. | Takács T, Czakó L, Morschl E, László F, Tiszlavicz L, Rakonczay Z, Lonovics J. The role of nitric oxide in edema formation in L-arginine-induced acute pancreatitis. Pancreas. 2002;25:277-282. [PubMed] |
| 10. | Chen HM, Shyr MH, Lau YT, Hwang TL, Chen MF. Leukocyte-endothelial adherence correlates with pancreatic nitric oxide production in early cerulein-induced pancreatitis in rats. Shock. 1998;10:218-222. [PubMed] |
| 11. | Rahman SH, Ammori BJ, Larvin M, McMahon MJ. Increased nitric oxide excretion in patients with severe acute pancreatitis: evidence of an endotoxin mediated inflammatory response? Gut. 2003;52:270-274. [PubMed] |
| 12. | Rau B, Bauer A, Wang A, Gansauge F, Weidenbach H, Nevalainen T, Poch B, Beger HG, Nussler AK. Modulation of endogenous nitric oxide synthase in experimental acute pancreatitis: role of anti-ICAM-1 and oxygen free radical scavengers. Ann Surg. 2001;233:195-203. [PubMed] |
| 13. | Tomaszewska R, Dembiński A, Warzecha Z, Ceranowicz P, Stachura J. Morphological changes and morphological-functional correlations in acute experimental ischemia/reperfusion pancreatitis in rats. Pol J Pathol. 2000;51:179-184. [PubMed] |
| 14. | Ayub K, Serracino-Inglott F, Williamson RC, Mathie RT. Expression of inducible nitric oxide synthase contributes to the development of pancreatitis following pancreatic ischaemia and reperfusion. Br J Surg. 2001;88:1189-1193. [PubMed] |
| 15. | Cuzzocrea S, Mazzon E, Dugo L, Serraino I, Centorrino T, Ciccolo A, Van de Loo FA, Britti D, Caputi AP, Thiemermann C. Inducible nitric oxide synthase-deficient mice exhibit resistance to the acute pancreatitis induced by cerulein. Shock. 2002;17:416-422. [PubMed] |
| 16. | Zhou ZG, Chen YD. Influencing factors of pancreatic microcirculatory impairment in acute panceatitis. World J Gastroenterol. 2002;8:406-412. [PubMed] |
| 17. | Leindler L, Morschl E, László F, Mándi Y, Takács T, Jármai K, Farkas G. Importance of cytokines, nitric oxide, and apoptosis in the pathological process of necrotizing pancreatitis in rats. Pancreas. 2004;29:157-161. [PubMed] |
| 18. | McDonald MC, Mota-Filipe H, Paul A, Cuzzocrea S, Abdelrahman M, Harwood S, Plevin R, Chatterjee PK, Yaqoob MM, Thiemermann C. Calpain inhibitor I reduces the activation of nuclear factor-kappaB and organ injury/dysfunction in hemorrhagic shock. FASEB J. 2001;15:171-186. [PubMed] |
| 19. | Duchen MR. Roles of mitochondria in health and disease. Diabetes. 2004;53 Suppl 1:S96-102. [PubMed] |
| 20. | Pi F, Badosa F, Sola A, Roselló Catafau J, Xaus C, Prats N, Gelpí E, Hotter G. Effects of adenosine on ischaemia-reperfusion injury associated with rat pancreas transplantation. Br J Surg. 2001;88:1366-1375. [PubMed] |
| 21. | Wakai A. Effect of adenosine on ischaemia-reperfusion injury associated with rat pancreas transplantation. Br J Surg. 2002;89:494. [PubMed] |
| 22. | Yamamoto H, Sugitani A, Kitada H, Arima T, Nishiyama Ki K, Shiiba M, Kawano R, Morisaki T, Nakafusa Y, Tanaka M. Effect of FR167653 on pancreatic ischemia-reperfusion injury in dogs. Surgery. 2001;129:309-317. [PubMed] |
| 23. | Larsen CM, Wadt KA, Juhl LF, Andersen HU, Karlsen AE, Su MS, Seedorf K, Shapiro L, Dinarello CA, Mandrup-Poulsen T. Interleukin-1beta-induced rat pancreatic islet nitric oxide synthesis requires both the p38 and extracellular signal-regulated kinase 1/2 mitogen-activated protein kinases. J Biol Chem. 1998;273:15294-15300. [PubMed] |
| 24. | Svensson AM, Sandler S, Jansson L. The blood flow in pancreatico-duodenal grafts in rats: inhibition of nitric oxide synthase preferentially decreases islet blood flow. Eur J Pharmacol. 1995;275:99-103. [PubMed] |
| 25. | Dobosz M, Wajda Z, Hać S, Myśliwska J, Bryl E, Mionskowska L, Roszkiewicz A, Myśliwski A. Nitric oxide, heparin and procaine treatment in experimental ceruleine-induced acute pancreatitis in rats. Arch Immunol Ther Exp (Warsz). 1999;47:155-160. [PubMed] |
| 26. | Benz S, Obermaier R, Wiessner R, Breitenbuch PV, Burska D, Weber H, Schnabel R, Mayer J, Pfeffer F, Nizze H. Effect of nitric oxide in ischemia/reperfusion of the pancreas. J Surg Res. 2002;106:46-53. [PubMed] |
| 27. | Yuan CH, Liu YF, Cheng Y, Zhao N, Li GC, Liang J, He SG. Protective effects of L-arginine on reperfusion injury after pancreaticoduodenal transplantation in rats. Hepatobiliary Pancreat Dis Int. 2004;3:349-354. [PubMed] |
| 28. | Vollmar B, Janata J, Yamauchi JI, Menger MD. Attenuation of microvascular reperfusion injury in rat pancreas transplantation by L-arginine. Transplantation. 1999;67:950-955. [PubMed] |
| 29. | Obermaier R, von Dobschuetz E, Muhs O, Keck T, Drognitz O, Jonas L, Schareck W, Hopt UT, Benz S. Influence of nitric oxide on microcirculation in pancreatic ischemia/reperfusion injury: an intravital microscopic study. Transpl Int. 2004;17:208-214. [PubMed] |
| 30. | Wolff DJ, Lubeskie A. Aminoguanidine is an isoform-selective, mechanism-based inactivator of nitric oxide synthase. Arch Biochem Biophys. 1995;316:290-301. [PubMed] |
| 31. | Eberhardt W, Kunz D, Pfeilschifter J. Pyrrolidine dithiocarbamate differentially affects interleukin 1 beta- and cAMP-induced nitric oxide synthase expression in rat renal mesangial cells. Biochem Biophys Res Commun. 1994;200:163-170. [PubMed] |
| 32. | Oddis CV, Finkel MS. NF-kappa B and GTP cyclohydrolase regulate cytokine-induced nitric oxide production by cardiac myocytes. Am J Physiol. 1996;270:H1864-H1868. [PubMed] |