修回日期: 2004-05-09
接受日期: 2004-05-13
在线出版日期: 2004-09-15
目的: 探讨肝硬化患者13C-美沙西丁肝功能呼气试验检测的特点.
方法: 肝硬化患者42例和正常人31名进行13C-美沙西丁呼气试验. 口服13C-美沙西丁75 mg, 收集检测并比较研究对象服药前、服药后10, 20, 30, 40, 50, 60, 80, 100, 120 min等10个时间段呼出气体中13CO2丰度(DOB)、代谢速率(MV)以及累积丰度(CD)等.
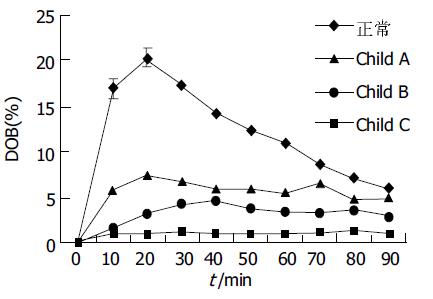
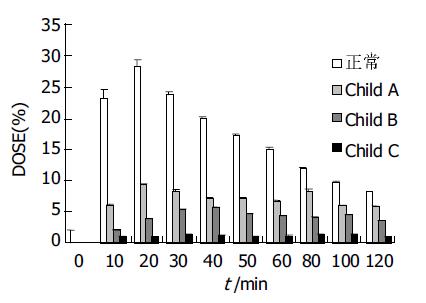
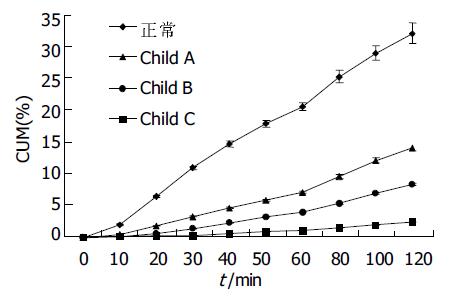
结果: 正常人呼出气体中 13CO2丰度有明显的峰值(20.3± 3.5%), 出现在服用13C-美沙西丁后20 min左右; Child A级患者为低平双峰峰值, 分别出现在20 min和80 min (7.5±1.8%和6.5±3.3%); Child B级患者峰值后移至 40 min (4.6±1.3%); 各组间峰值差别均有显著统计学意义(P <0.05), Child C级患者无峰值. 正常人和Child A级患者代谢速率最高峰出现在20 min (28.8±5.3%和9.4±2.4%), 差别有统计学意义(P <0.05), 而Child B级患者代谢速率普遍降低且无峰值, Child C级患者代谢速率极低. 正常人、Child A级、Child B级、Child C级患者120 min 13CO2累积呼出丰度依次分别为31.2±4.5%, 13.8±3.7%, 8.2±2.2%, 2.4±0.8%, 各组间差异显著(P <0.05).
结论: 13C-美沙西丁呼气试验检测能准确、直观地反映肝硬化患者肝功能状况.
引文著录: 万荣, 吴云林, 王虹, 瞿晴, 张曙. 13C-美沙西丁呼气试验判断肝硬化患者肝功能. 世界华人消化杂志 2004; 12(9): 2147-2149
Revised: May 9, 2004
Accepted: May 13, 2004
Published online: September 15, 2004
AIM: To investigate the characteristics of the 13C-methacetin breath test (13C-MBT) as a tool to monitor hepatic function of patients with liver cirrhosis.
METHODS: Forty-two patients with liver cirrhosis and thirty-one age- and sex-matched healthy controls underwent 13C-MBT.13C-methacetin (75 mg) was given orally, and the levels of 13CO2 delta over baseline (DOB), metabolisation velocity (MV) and cumulative percentage doses (CD) before and 10, 20, 30, 40, 50, 60, 80, 100 and 120 min after substrate administration breath samples was determined and compared, by non-dispersive infrared spectrometry.
RESULTS: The 13CO2 peak percent doses of healthy controls, Child class A and Child class B patients were 20.3 ±3.5%, 7.5±1.8% or 6.5±3.3% and 4.5±1.3%, and occurred at 20 min, 20 or 80 min and 40 min, respectively. The differences of13CO2 peak percent doses among healthy controls, Child class A and Child class B patients were obvious (P <0.05). No 13CO2peak percent doses occurred in Child class C patients. The differences of 13CO2 peak metabolic velocity were obvious (P <0.05) between healthy controls (28.8±5.3%) and Child class A patients (9.4±2.4%). No 13CO2 peak metabolic velocity occurred in Child class B and Child class C patients. The 13CO2 cumulative percentage doses of healthy controls, Child class A , Child class B and Child class C patients at 120 min after substrate administration were 31.2±4.5%, 13.8±3.7%, 8.2±2.2% and 2.4±0.8% (P <0.05), respectively.
CONCLUSION: The severity of liver damage can be directly and effectively evaluated by 13C-MBT in patients with cirrhosis.
- Citation: Wan R, Wu YL, Wang H, Qu Q, Zhang S. Evaluation of hepatic function with 13C-methacetin breath test in patients with liver cirrhosis. Shijie Huaren Xiaohua Zazhi 2004; 12(9): 2147-2149
- URL: https://www.wjgnet.com/1009-3079/full/v12/i9/2147.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v12.i9.2147
以往应用的肝功能检测试验如吲哚靛氰绿(ICG), 磺溴肽钠(BSP)等, 虽能反映肝脏储备功能, 但由于操作复杂, 影响因素多, 以及潜在的不良反应, 限制了他们在临床上的广泛应用. 稳定性同位素13C标记底物已应用于临床肝功能检测 [1-11]. 现就13C-美沙西丁呼气试验对肝硬化患者肝功能检测的特点进行分析.
经解释和征得本人同意后, 列为研究对象. 无肝病体检者31例, 其中男19例, 女12例, 年龄22-70(平均37±6.7岁). 肝硬化患者51例(肝炎后肝硬化39例, 酒精性肝硬化7例, 血吸虫性肝硬化2例, 不明原因3例), 男28例, 女23例, 年龄23-71(平均40±2.7岁). 肝硬化诊断标准符合2000年西安全国传染病与寄生虫病学术会议修订的肝硬化诊断标准, 均经肝组织活检和/或CT, B超及临床生化肝功能检查证实并临床Child-Pugh 分级. 13C-美沙西丁(75 mg)和IRIS分析仪(infra red isotope analyser 红外线同位素能谱分析仪)由德国WAGNER公司生产.
隔夜空腹, 收集零时气100 mL, 服用13C-美沙西丁75 mg后, 于安静坐位状态下分别收集10、20、30、40、50、60、80、100、120 min 等时间段呼出气体各100 mL, 通过13C红外线能谱分析仪检测并标明其各时间段DOB值、代谢速率值(Dose/h)、累积丰度值(cum dose )等各项指标. 肝硬化患者采用Child-Pugh 分级, 其中Child A级12例, Child B级15例, Child C级24例, 分别计算各组患者不同时间段DOB, Dose/h, Cum Dose均数±标准差值.
统计学处理 数据统计选用SAS软件包t 检验, 方差分析.
随肝功能Child分级越差, 曲线越为低平. 正常人呼出气体(样品袋中气体)中 13CO2丰度曲线有明显的峰值(20.3±3.5%), 出现在服用13C-美沙西丁后20 min; Child A级患者为低平双峰曲线, 峰值分别出现在20 min和80 min(7.5±1.8%和6.5±3.3%); Child B级患者曲线更为低平, 峰值明显后移至40 min (4.6±1.3%); 各组间峰值差别均有显著统计学意义(P <0.01). Child C级患者曲线接近基线且无峰值(图1).
正常人和Child A级患者代谢速率最高峰均出现在20 min (28.8±5.3%和9.4±2.4%), 各组间峰值差别均有显著性意义(P <0.01, 图2). Child B级代谢速率普遍降低且无峰值. Child C级患者代谢速率极低, 接近基底线.
正常人累积丰度(CD)曲线斜率明显高于其他各组. 随肝硬化患者肝功能Child 分级不断加重, 曲线斜率相应变小. 各组120 min 13CO2累积呼出丰度依次分别为31.2±4.5%, 13.8±3.7%, 8.2±2.2%, 2.4±0.8%, 各组间均有显著性差异(P <0.05, 图3).
肝细胞的生物转化功能绝大部分都在滑面内质网(微粒体)完成, 包括氧化、还原、水解及结合共4种方式. 微粒体将药物及毒物经过氧化、还原、水解及结合等方式转化为有极性的水溶性物质. 每一活的健康肝细胞都有完善的内质网(微粒体)系统, 当然具备完善的加单氧酶系及其所表现出的完备的生物转化功能. 国内外已经开展了多种同位素检测肝功能法[12-18]. 本研究采用的Methacetin(乙酰对甲氧基苯胺)是其中的一种, 为非那西汀的衍生物, 经由肝微粒体酶系可以很快地氧化脱甲基化, 最终产物13CO2由肺呼出. 13C标记的底物(13C-美沙西丁)检测肝功能机制: 由摄取-胃排空-吸收-转移至肝脏-肝脏代谢-产生13CO2呼出, 通过检测不同时间段呼出气体中13CO2的丰度(DOB)、代谢速率(MV)和累积丰度(CD)等, 反映肝微粒体的去甲基的能力, 从而评估患者肝功能状态[17-19].
DOB曲线上每一点的数值代表着该时刻呼出气 (样品袋中气体) 的13CO2的丰度(含量). 本研究显示, 正常人呼出13CO2的丰度峰值明显, 时间出现在服药后的20 min左右, 曲线有明显的上升段和下降段. 依肝损程度的不断加重, 13CO2呼出的峰值越来越低, 峰值出现时间也有相应的延迟. 由此可以推测, 随病损程度的不断加重, 肝细胞微粒体的去甲基功能不断减弱, 处理13C标记的底物的能力也相应减低, 因而表现出呼出气体中13CO2 最高含量下降及其出现时间的延迟.
代谢速率曲线上每一点的速率单位为Dose/h(%), 即在某一时刻13CO2 的呼出速率, 具体表示为某一时刻呼出的 13CO2 的丰度占全部应呼出量的13CO2丰度的百分比. 他是由IRIS软件系统根据每一患者的身高、体重计算其体表面积和CO2排出量, 并在此基础上依据服入体内的底物的质量、分子质量及原子价, 以及13C/12C的PDB常数等计算得出的. 本研究同样显示, 随病损的不断加重, 最大呼出速率曲线的峰值也有相应程度的下降, 直至在Child B和Child C级时的峰值不明显, 各组间(除Child B与Child C外)呼出速率峰值间差别均有显著性差异(P < 0.01). 由此可见, 通过检测13CO2呼出速率及观察其呼出速率曲线的变化可以量化并直观地反映出患者肝功能的状态.
累积丰度是指在某一时刻前累积呼出的13CO2的丰度总和, 单位表示为%, 即在某一时刻前呼出了百分之多少的13CO2. 他实际上是指某一时刻前代谢速率曲线下面的面积, 对此面积进行微积分处理即得到该面积的精确数值, 他代表着该时刻的13CO2累积丰度. 本研究得出, 各组间13CO2累积丰度在30 min (10.8±2.2%, 6.4±0.5%, 3.3±1.1%, 1.4±0.5%, 0.3±0.1%), 60 min (20.3±3.2%, 11.4±1.8%, 6.9±2.2%, 3.9±0.9%, 1.1±0.9%), 120 min (31.2±4.5%, 16.9±5.1%, 13.8±3.7%, 8.2±2.2%, 2.4±0.8%)差别均有统计学意义(P <0.01或P <0.05).
临床肝硬化分级是靠多个指标的累加计分来完成, 以求较完整的反映患者肝功能. 本研究中Mvmax值(代谢速率峰值与正常值的比值)、CUM值(13CO2累积呼出丰度与正常值的比值)均为精确数值, 与病情严重程度相关, 随病情加重, 3种指标值均呈下降趋势, 与临床肝功能分级相比, 13C-美沙西丁呼气试验在数字化分级的基础上对肝细胞功能进行准确的评定, 因而更为直接和准确[19]. 本研究13C肝细胞功能检测给出了三类动力曲线, 曲线量化和直观, 通过对各类曲线中不同程度的肝损及其不同时相数值之间的差别分析, 能准确、直观地反映肝内肝细胞即时的储备功能及代偿状况.
| 1. | Ishii T, Furube M, Hirano S, Takatori K, Iida K, Kajiwara M. Evaluation of 13C-phenylalanine and 13C-tyrosine breath tests for the measurement of hepatocyte functional capacity in patients with liver cirrhosis. Chem Pharm Bull (Tokyo). 2001;49:1507-1511. [PubMed] [DOI] |
| 2. | Kobayashi T, Kubota K, Imamura H, Hasegawa K, Inoue Y, Takayama T, Makuuchi M. Hepatic phenylalanine metabolism measured by the [13C]phenylalanine breath test. Eur J Clin Invest. 2001;31:356-361. [PubMed] [DOI] |
| 3. | Saadeh S, Behrens PW, Parsi MA, Carey WD, Connor JT, Grealis M, Barnes DS. The utility of the 13C-galactose breath test as a measure of liver function. Aliment Pharmacol Ther. 2003;18:995-1002. [PubMed] [DOI] |
| 4. | Park GJ, Katelaris PH, Jones DB, Seow F, Le Couteur DG, Ngu MC. Validity of the 13C-caffeine breath test as a noninvasive, quantitative test of liver function. Hepatology. 2003;38:1227-1236. [PubMed] [DOI] |
| 5. | Ishii Y, Suzuki S, Kohno T, Aoki M, Kohno T, Ito A, Takayama T, Asai S. L-[1-13C] phenylalanine breath test reflects histological changes in the liver. J Surg Res. 2003;114:120-125. [PubMed] [DOI] |
| 6. | Parra D, González A, García-Villarreal L, Martínez JA. Methodological characterization of the 2-keto [1-13C]isocaproate breath test to measure in vivo human mitochondrial function: application in alcoholic liver disease assessment. Alcohol Clin Exp Res. 2003;27:1293-1298. [PubMed] [DOI] |
| 7. | Ishii Y, Suzuki S, Kohno T, Aoki M, Kohno T, Ito A, Takayama T, Asai S. L-[1-13C] phenylalanine breath test reflects phenylalanine hydroxylase activity of the whole liver. J Surg Res. 2003;112:38-42. [PubMed] [DOI] |
| 8. | Di Campli C, Angelini G, Armuzzi A, Nardo B, Zocco MA, Candelli M, Santoliquido A, Cavallari A, Bernardi M, Gasbarrini A. Quantitative evaluation of liver function by the methionine and aminopyrine breath tests in the early stages of liver transplantation. Eur J Gastroenterol Hepatol. 2003;15:727-732. [PubMed] [DOI] |
| 9. | Ishii Y, Asai S, Kohno T, Ito A, Iwai S, Ishikawa K. Recovery of liver function in two-third partial hepatectomized rats evaluated by L-[1-13C]phenylalanine breath test. Surgery. 2002;132:849-856. [PubMed] [DOI] |
| 10. | Tugtekin I, Wachter U, Barth E, Weidenbach H, Wagner DA, Adler G, Georgieff M, Radermacher P, Vogt JA. Phenylalanine kinetics in healthy volunteers and liver cirrhotics: implications for the phenylalanine breath test. Am J Physiol Endocrinol Metab. 2002;283:E1223-E1231. [PubMed] [DOI] |
| 11. | Caubet MS, Laplante A, Caillé J, Brazier JL. [13C]aminopyrine and [13C]caffeine breath test: influence of gender, cigarette smoking and oral contraceptives intake. Isotopes Environ Health Stud. 2002;38:71-77. [PubMed] [DOI] |
| 12. | Ishii Y, Ishikawa K, Asai S. [Evaluation of liver function with 13C-labelled amino acid using hepatectomized rat model]. Nihon Yakurigaku Zasshi. 2002;120:101-106. [PubMed] [DOI] |
| 13. | Giannini E, Fasoli A, Chiarbonello B, Malfatti F, Romagnoli P, Botta F, Testa E, Polegato S, Fumagalli A, Testa R. 13C-aminopyrine breath test to evaluate severity of disease in patients with chronic hepatitis C virus infection. Aliment Pharmacol Ther. 2002;16:717-725. [PubMed] [DOI] |
| 14. | Mana F, Georges B, Reynaert H, Ham HR, Urbain D. Evaluation of the 13C-aminopyrine breath test using nondispersive infrared spectrometry. Acta Gastroenterol Belg. 2000;63:328-330. [PubMed] |
| 15. | Armuzzi A, Marcoccia S, Zocco MA, De Lorenzo A, Grieco A, Tondi P, Pola P, Gasbarrini G, Gasbarrini A. Non-Invasive assessment of human hepatic mitochondrial function through the 13C-methionine breath test. Scand J Gastroenterol. 2000;35:650-653. [PubMed] [DOI] |
| 16. | Candelli M, Armuzzi A, Nista EC, Fini L, Gasbarrini G, Gasbarrini A. 13C-methacetin breath test for monitoring hepatic function in cirrhotic patients before and after liver transplantation. Aliment Pharmacol Ther. 2004;19:243. [PubMed] [DOI] |
| 17. | Candelli M, Cazzato IA, Nista EC, Pignataro G, Gasbarrini A. 13C-methacetin breath test and oxygen supply. Aliment Pharmacol Ther. 2003;18:1176. [DOI] |
| 18. | Lara Baruque S, Razquin M, Jimenez I, Vazquez A, Gisbert JP, Pajares JM. 13C-phenylalanine and 13C-methacetin breath test to evaluate functional capacity of hepatocyte in chronic liver disease. Dig Liver Dis. 2000;32:226-232. [PubMed] [DOI] |
| 19. | Zipprich A, Meiss F, Steudel N, Sziegoleit U, Fleig WE, Kleber G. 13C-Methacetin metabolism in patients with cirrhosis: relation to disease severity, haemoglobin content and oxygen supply. Aliment Pharmacol Ther. 2003;17:1559-1562. [PubMed] [DOI] |