修回日期: 2004-07-09
接受日期: 2004-07-22
在线出版日期: 2004-09-15
目的: 探讨细胞间信息传递因子NO在胃黏膜遭受应激损伤时的变化及与细胞凋亡发生的关系.
方法: 原位末端标记(TUNEL)法检测大鼠水浸-束缚应激(WRS)结束前后不同时间点细胞凋亡的发生; 免疫组化的方法检测胃黏膜组织nNOS/iNOS蛋白表达的变化; 检测不同剂量的NO合成抑制剂L-NAME对细胞凋亡的影响.
结果: iNOS蛋白表达与细胞凋亡的变化呈显著正相关; nNOS蛋白表达与细胞凋亡变化呈负相关; 与对照组比较, 小剂量L-NAME(2.0 mg/kg)可降低凋亡细胞发生率34.6%(P <0.01); 大剂量L-NAME(20.0 mg/kg)明显增加凋亡细胞发生率25.8% (P <0.05).
结论: 正常生理状态下及应激初期, 胃黏膜以nNOS表达为主. 若应激源持续存在, nNOS表达受抑制, iNOS表达增强, 诱导合成大量NO, 有明显细胞毒作用, 促进细胞凋亡发生.
引文著录: 刘婧, 李兆申, 宛新建, 王雯. 大鼠应激性溃疡胃黏膜nNOS/iNOS表达对细胞凋亡的影响. 世界华人消化杂志 2004; 12(9): 2127-2130
Revised: July 9, 2004
Accepted: July 22, 2004
Published online: September 15, 2004
AIM: To determine the effect of different type of NOS expression on gastric mucosal cell apoptosis during stress ulcer in mice.
METHODS: Apoptotic cells were quantitated in gastric mucosa by terminal deoxynucleatidyl transferase mediated dUTP nick and labelling (TUNEL) techniques. The expression of nNOS/iNOS proteins was detected by immunohistochemical method. The effects of different dose of nitric oxide inhibitor NG-nitro-L-arginine methyl ester (L-NAME) on gastric mucosal apoptosis was investigated in immersion-restraint stress model of mice.
RESULTS: The expression of iNOS was highly correlated with the apoptotic cell quantities of gastric mucosa. In contract to the high dose of L-NAME (20.0 mg/kg) that increased the quantity of apoptosis 25.8% (P <0.05), small dose of L-NAME (2.0 mg/kg) decreased the quantity of apoptosis 34.6% (P <0.01).
CONCLUSION: The expression of NO is a double-edged sword, a low amount of NO produced by neuronal forms of NOS performs many physiological functions. On the contrary, higher concentration of NO produced by inducible form of NOS induces the epithelial cell apoptosis under stress condition.
- Citation: Liu J, Li ZS, Wan XJ, Wang W. Effect of nNOS/iNOS expression on cell apoptosis during stress ulcer of gastric mucosa in mice. Shijie Huaren Xiaohua Zazhi 2004; 12(9): 2127-2130
- URL: https://www.wjgnet.com/1009-3079/full/v12/i9/2127.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v12.i9.2127
正常胃黏膜细胞凋亡与细胞增生共同调节胃黏膜结构和功能的稳态[1-4]. 在胃黏膜遭受到应激性损伤时, 胃黏膜细胞相互之间通过局部的信息传递所做出的快速、应激性反应, 可能是维护细胞功能的重要因素. NO是一种具有广泛的生物学效应的小分子化合物, 其基本生物学作用之一是细胞间信息交换, 他允许一个细胞产生的信号迅速地作用于相邻的靶细胞发挥生物效应, 而不受释放、摄取等机制的调控. 目前研究发现NO能通过不同途径对胃黏膜细胞凋亡进行精细调节[5-6]. 本研究检测SU发生过程中细胞凋亡的发生以及与凋亡密切相关的nNOS/iNOS表达的变化.
体质量200-220 g♂Sprague-Dwley(SD)大鼠, 1-2月月龄(上海西普尔-必凯实验动物有限公司提供). 参考Brodie (Gastroenterology 1960;38:353)的方法制备水浸-束缚应激(water-immersion and restraint stress, WRS)溃疡模型, WRS 2, 3.5, 结束后2, 5, 8, 12, 24 h (7组, 每组8只). 对照组为没有经过任何处理的SD大鼠, 6只. A: 小剂量L-NAME (2.0 mg/kg)组; B: 小剂量D-NAME(2.0 mg/kg)组: C: 大剂量L-NAME (20.0 mg/kg)组; D:大剂量D-NAME(20.0 mg/kg)组: 以上各组药物用生理盐水稀释至1 mL, 应激前30 min ip. 对照组: 生理盐水1 mL, 应激前30 min ip. WRS结束后2 h处死大鼠, 每组SD大鼠8只, 共5组. SD大鼠在相应时间在300 mg/kg水合氯醛麻醉下, 行灌流固定. 取腺胃区组织块0.5 cm×0.5 cm, 经后固定、脱水后在恒温箱切片机内作冰冻切片, 片厚10 mm.
原位末端标记(TUNEL)法原位检测凋亡. 冰冻切片在40 g/L多聚甲醛中固定20 min, 0.01 mol/LPBS洗5 min×3次, 250 mL/L冰醋酸洗10 min×1次, 0.01 mol/L PBS洗5 min×3次, Protease K (20 mg/L) (promega公司提供)37.0 ℃孵育30 min, 40 g/L多聚甲醛固定5 min, 0.01 mol/L PBS洗5 min×3次, 双蒸水洗5 min×4次, 空气中凉干, 加标记液(Promega公司提供)37.0 ℃ 1-1.5 h, 0.05 mol/L PBS洗5 min ×3次, 碱性磷酸酶标记的地高辛抗体片段(Boeringer公司提供)1:1 000稀释, 置室温下4 h, NBT和BCIP(华美试剂公司提供)显色3 h. 利用nNOS/iNOS免疫组化试剂盒(北京中山生物-试剂公司提供), 采用ABC法检测nNOS/iNOS的表达. NOS免疫组化片, 光镜下每例组织观察3张切片, 其中深棕黄色为强阳性反应(+++); 黄色为中等阳性反应(++); 浅黄色为阳性反应(+); 黄灰色为可疑阳性反应(-); 着色与背景灰色相同为阴性反应(-). 将TUNEL染色片在BH-2显微镜下, 固定输入条件, 将视野内图像通过彩色摄像机输入A6300图像采集卡, 应用通用颗粒图像分析系统, 对采集图像进行二值灰度分割, 得到所需信号的半灰度目标图像, 以人-机交换的方式, 求出单位面积内给定灰度值以下的阳性颗粒的颗粒的面积值(像数点). 将免疫组化染色片在0.5 cm×1.0 cm的样品截面上, 用计算机图像处理系统进行定量灰度扫描, 求出给定面积内的灰度值, 将各点的灰度值换算为可用参数(灰度越大, 在监视器上越亮, 故染色越深, 在监视器上颜色越暗, 灰度级数越小)每只大鼠3张切片, 每个时间点5只大鼠, 并进行t 检验.
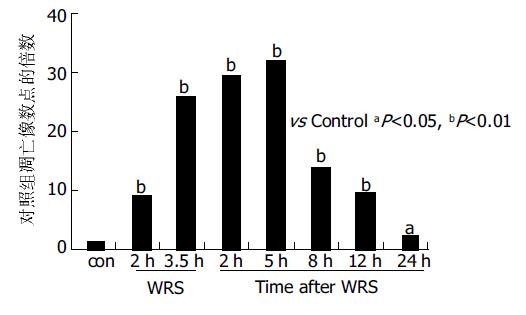
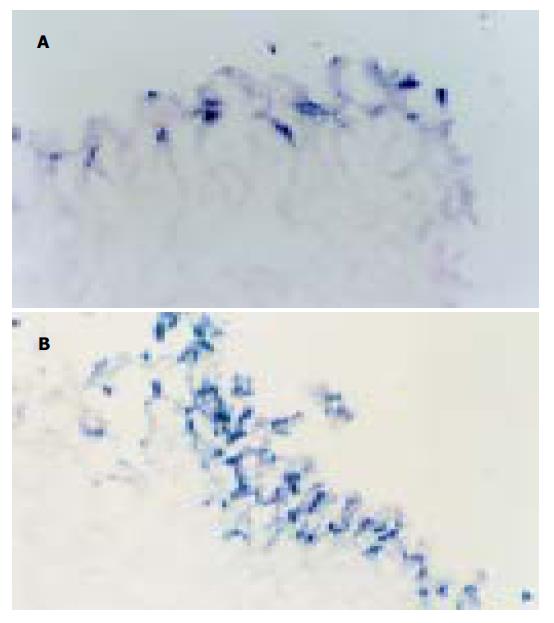
正常大鼠胃黏膜上皮散在TUNEL-细胞. WRS 2 h TUNEL细胞较对照组明显增多(P <0.01); WRS3.5 h/WRS后5 h阳性细胞数量达高峰. WRS后8 h随着时间延长TUNEL细胞逐渐减少, 但到WRS24 h仍高于正常水平(P <0.05图1, 图2A, B).
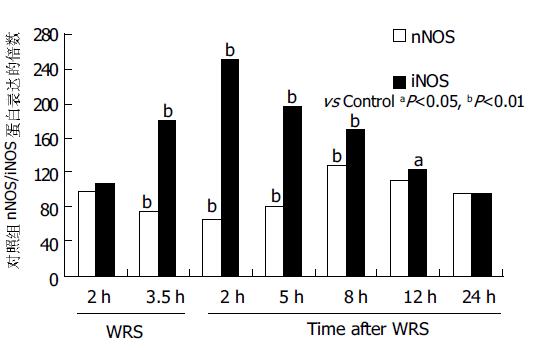
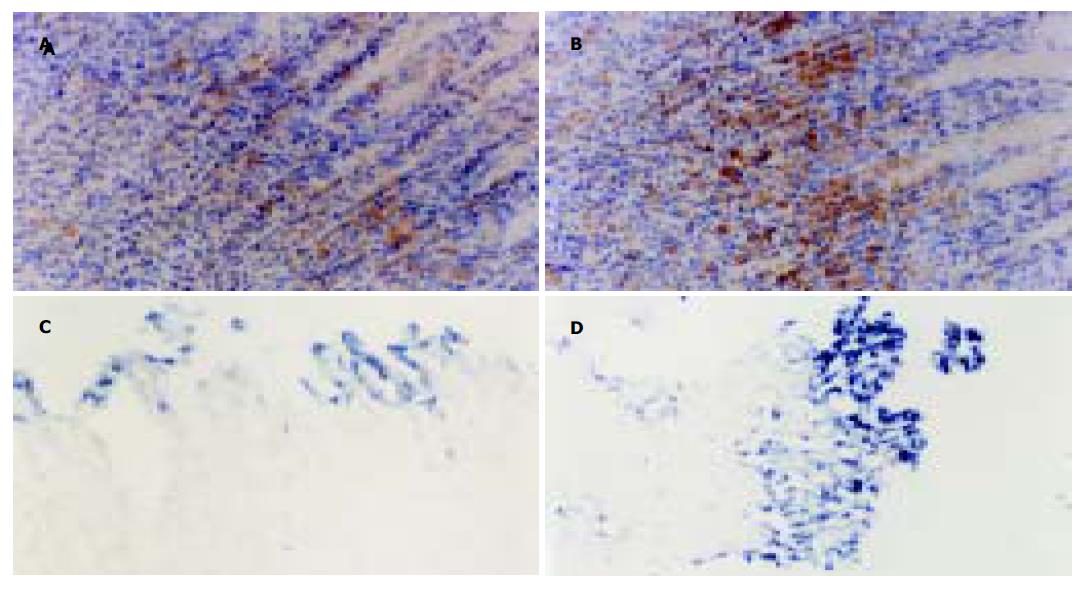
正常胃黏膜组织中, nNOS蛋白主要在胃腺区及固有层内呈中等阳性表达, 为胞质棕黄染色. iNOS蛋白仅在靠近胃小凹部有阳性表达. WRS2 h nNOS表达未见明显减少, WRS3.5 h/WRS后5 h表达逐渐减少, 而iNOS表达逐渐增多, 在整个胃黏膜层呈强阳性表达. WRS后8 h nNOS表达增多, iNOS的表达逐渐减少, 但在上皮层仍呈强阳性表达. WRS后24 h, 二者基本恢复至正常水平. 相关分析提示: iNOS蛋白表达与细胞凋亡的变化呈显著正相关(g = 0.9 304, P <0.01); nNOS蛋白表达与细胞凋亡变化呈负相关(g=-0.7 154, P <0.05, 图3, 图4A, B).
小剂量L-NAME (2.0 mg/kg)可适当减轻大鼠WRS结束后2 h胃黏膜损伤程度, 计算单位面积内TUNEL细胞的像数点, 较生理盐水组降低34.6%(P <0.01), 大剂量L-NAME(20.0 mg/kg)明显加重胃黏膜损伤程度, TUNEL细胞数较生理盐水组增加25.8% (P <0.05). 而两种剂量的D-NAME对黏膜损伤较生理盐水组均无显著性差异(图4C, D).
胃黏膜细胞凋亡生和增生之间的平衡是维持胃黏膜正常结构和功能的基础. 近年发现, 凋亡有可能参与了急性胃黏膜损伤的过程, 同时, 他也是刺激、促进细胞增生的一个重要因素[7]. 应激因素作用于胃黏膜, 细胞要对应激性损伤作出快速反应, 最终决定细胞是生存或是死亡, 作用方式简捷、迅速的NO可能在此过程中发挥了重要的作用. 不同类型的NOS在胃肠道不同细胞内的定位与NOS对胃黏膜生理、病理功能的调节密切相关[8]. 我们发现, nNOS在正常大鼠胃黏膜的胃小凹部、胃腺部均有表达, 而iNOS仅在胃小凹部有弱表达. 提示, 正常生理状态下, nNOS可能发挥着更广泛的作用, 对于胃肠道正常生理功能的维持和调节有重要意义.
近来研究发现NO参与了多种细胞的凋亡过程, NO与胃上皮细胞凋亡之间的关系尚不明确, Watanabe et al[9]的研究证实Hp感染产生的代谢产物LPS等诱导iNOS大量产生, 后者通过氧化应激造成DNA损伤进而导致大量胃上皮细胞凋亡. 但Fiorucci et al认为, aspirin通过ICE类蛋白酶途径诱导细胞凋亡, 适当剂量的NO可减轻细胞凋亡的发生. 我们发现, 应激2 h后nNOS的表达仍接近正常水平, 这可能是机体对应激性损伤的一种保护性代偿反应. 大量研究证实, 一定剂量的NO可松弛血管平滑肌, 增加碳酸盐、黏液的分泌, 抑制胃酸的分泌, 从而对局部胃黏膜产生细胞保护作用[10-15]. 但在应激源持续存在的情况下, WRS3.5 h/WRS后5 h 大量胃上皮细胞发生凋亡, nNOS的表达明显低于正常值, 而iNOS的表达逐渐增强, 并且不仅限于胃上皮小凹区, 胃腺区的表达也明显增强, WRS结束后12 h细胞凋亡逐渐减少, iNOS的表达也逐渐降低, 但在凋亡发生明显的胃上皮细胞区, 表达仍较显著. 小剂量NO合成抑制剂L-NAME(2.0 mg/kg)ip后[16-17], 部分抑制NO的合成, WRS后2, 5, 8 h黏膜损伤程度、细胞凋亡发生率较未用药组明显减少. 小剂量D-NAME无此作用. 但大剂量的L-NAME(20.0 mg/kg) ip, 则加重溃疡的形成, WRS2 h胃黏膜即出现大量出血点, TUNEL标记的凋亡细胞和坏死细胞都明显增多. 这与许多报道一致, 其机制可能源于NO在机体内广泛、复杂的病理生理效应, 完全抑制NO的生成, 必然使其扩张血管、调节黏膜血流量, 抑制血小板聚集、调节递质释放等作用消失, ET-1等缩血管物质生成增多, 胃黏膜局部缺血、缺氧加重, 黏膜损伤加重[18-21]. 大剂量的D-NAME无此作用. 由此可见, 应激性溃疡发生过程中, 不同类型NOS随时间的表达变化产生一定剂量的NO与细胞凋亡的发生有一定关系. 正常生理状态下及应激初期, nNOS的表达可能对细胞产生保护作用, 对细胞的增生和凋亡进行精细调节. 已有研究证实, nNOS可通过cGMP-依赖机制, 调节凋亡发生过程中的关键蛋白酶-caspase的磷酸化和去磷酸化过程抑制其催化活性, 或通过抑制其活性亚硝酰基而阻止bcl-2分裂, 以及调节炎性细胞因子ET-1等和其相应受体的相互作用, 抑制线粒体释放细胞色素C, 最终发挥凋亡抑制效应[22-26].
应激源持续存在的情况下, nNOS表达受抑制, 作用逐渐减弱, 而iNOS诱导合成大量的NO, 有明显细胞毒作用, 促进细胞凋亡发生. NO的病理生理作用极其复杂, 其对细胞凋亡的调节也可能通过不同的途径: (1)NO能直接损伤细胞的DNA, 诱导细胞凋亡; (2)NO与O2-结合可生成ONOO-, 后者可直接或通过分解成许多小分子毒性更强的物质例如NO2、OH-等对靶细胞产生直接的损伤作用; (3)NO通过cGMP/非cGMP依赖方式等信号通路作用于细胞凋亡基因, 启动凋亡程序, 调节凋亡相关基因的表达, 诱导细胞凋亡. 有研究表明, NO促进Fas基因的表达可造成血管平滑肌细胞的凋亡[27]. NO供体如S-硝基谷胱甘肽等能分别下调Bcl-2的表达和上调Bax表达, 诱导细胞凋亡; (4)与其他细胞因子如: IFN、TNF、IL、脂多糖或细菌内毒素等相互作用, 或刺激单核巨噬细胞系统释放细胞因子而杀伤靶细胞, 诱导靶细胞凋亡[28-30]; (5)NO与FES的酶结合生成铁硝基化合物, 可影响线粒体功能, 干扰能量代谢, 诱导细胞凋亡. 总之, NO究竟发挥哪种作用取决于NOS的类型、NO释放的动力学及靶细胞对NO的生物应答反应. nNOS和iNOS在调节细胞凋亡过程中发挥不同的作用.
| 1. | Maity P, Biswas K, Roy S, Banerjee RK, Bandyopadhyay U. Smoking and the pathogenesis of gastroduodenal ulcer--recent mechanistic update. Mol Cell Biochem. 2003;253:329-338. [PubMed] [DOI] |
| 2. | Sougioultzis S, Foukas PG, Tzivras M, Kourtessas D, Gorgoulis VG, Davaris P, Archimandritis AJ. Alterations in the proliferating compartment of gastric mucosa during Helicobacter pylori infection: the putative role of epithelial cells expressing p27(kip1). Mod Pathol. 2003;16:1076-1085. [PubMed] [DOI] |
| 3. | Ohkura Y, Furihata T, Kawamata H, Tabuchi M, Kubota K, Terano A, Sakai T, Fujimori T. Evaluation of cell proliferation and apoptosis in Helicobacter pylori gastritis using an image analysis processor. Gastric Cancer. 2003;6:49-54. [PubMed] [DOI] |
| 4. | Cho CH, Wu KK, Wu S, Wong TM, So WH, Liu ES, Chu KM, Shin VY, Ye YN, Wong BC. Morphine as a drug for stress ulcer prevention and healing in the stomach. Eur J Pharmacol. 2003;460:177-182. [PubMed] [DOI] |
| 5. | Miyazawa M, Suzuki H, Masaoka T, Kai A, Suematsu M, Nagata H, Miura S, Ishii H. Suppressed apoptosis in the inflamed gastric mucosa of Helicobacter pylori-colonized iNOS-knockout mice. Free Radic Biol Med. 2003;34:1621-1630. [PubMed] [DOI] |
| 6. | Kim JM, Kim JS, Jung HC, Oh YK, Chung HY, Lee CH, Song IS. Helicobacter pylori infection activates NF-kappaB signaling pathway to induce iNOS and protect human gastric epithelial cells from apoptosis. Am J Physiol Gastrointest Liver Physiol. 2003;285:G1171-G1180. [PubMed] [DOI] |
| 7. | Morini G, Grandi D, Schunack W. Ligands for histamine H(3) receptors modulate cell proliferation and migration in rat oxyntic mucosa. Br J Pharmacol. 2002;137:237-244. [PubMed] [DOI] |
| 9. | Watanabe S, Takagi A, Koga Y, Kamiya S, Miwa T. Helicobacter pylori induces apoptosis in gastric epithelial cells through inducible nitric oxide. J Gastroenterol Hepatol. 2000;15:168-174. [PubMed] [DOI] |
| 10. | Phillipson M. Acid transport through gastric mucus. Ups J Med Sci. 2004;109:1-24. [PubMed] [DOI] |
| 11. | Brzozowski T. Experimental production of peptic ulcer, gastric damage and cancer models and their use in pathophysiological studies and pharmacological treatment--Polish achievements. J Physiol Pharmacol. 2003;54 Suppl 3:99-126. [PubMed] |
| 12. | Gyires K. Neuropeptides and gastric mucosal homeostasis. Curr Top Med Chem. 2004;4:63-73. [PubMed] [DOI] |
| 13. | Miyoshi M, Kasahara E, Park AM, Hiramoto K, Minamiyama Y, Takemura S, Sato EF, Inoue M. Dietary nitrate inhibits stress-induced gastric mucosal injury in the rat. Free Radic Res. 2003;37:85-90. [PubMed] [DOI] |
| 14. | Kaneko H, Taché Y, Kusugami K. Importance of medullary thyrotropin-releasing hormone in brain-gut circuits regulating gastric integrity: preclinical studies. J Gastroenterol. 2002;37 Suppl 14:128-132. [PubMed] [DOI] |
| 15. | Ancha H, Ojeas H, Tedesco D, Ward A, Harty RF. Somatostatin-induced gastric protection against ethanol: involvement of nitric oxide and effects on gastric mucosal blood flow. Regul Pept. 2003;110:107-113. [PubMed] [DOI] |
| 16. | Oztürk H, Kara IH, Otçu S, Kilinc N, Yagmur Y. Influence of L-NAME and L-Arg on ischaemia-reperfusion induced gastric mucosa damage. Acta Gastroenterol Belg. 2002;65:150-154. [PubMed] |
| 17. | Konturek PC, Brzozowski T, Meixner H, Ptak A, Hahn EG, Konturek SJ. Central and peripheral neural aspects of gastroprotective and ulcer healing effects of lipopolysaccharides. J Physiol Pharmacol. 2001;52:611-623. [PubMed] |
| 18. | Phillipson M, Henriksnäs J, Holstad M, Sandler S, Holm L. Inducible nitric oxide synthase is involved in acid-induced gastric hyperemia in rats and mice. Am J Physiol Gastrointest Liver Physiol. 2003;285:G154-G162. [PubMed] [DOI] |
| 19. | Neu B, Puschmann AJ, Mayerhofer A, Hutzler P, Grossmann J, Lippl F, Schepp W, Prinz C. TNF-αlpha induces apoptosis of parietal cells. Biochem Pharmacol. 2003;65:1755-1760. [PubMed] [DOI] |
| 20. | Konturek PC, Brzozowski T, Meixner H, Ptak A, Hahn EG, Konturek SJ. Central and peripheral neural aspects of gastroprotective and ulcer healing effects of lipopolysaccharides. J Physiol Pharmacol. 2001;52:611-623. [PubMed] |
| 21. | Peskar BM. Neural aspects of prostaglandin involvement in gastric mucosal defense. J Physiol Pharmacol. 2001;52:555-568. [PubMed] |
| 23. | Souza MH, Lemos HP, Oliveira RB, Cunha FQ. Gastric damage and granulocyte infiltration induced by indomethacin in tumour necrosis factor receptor 1 (TNF-R1) or inducible nitric oxide synthase (iNOS) deficient mice. Gut. 2004;53:791-796. [PubMed] [DOI] |
| 24. | Tatemichi M, Ogura T, Sakurazawa N, Nagata H, Sugita M, Esumi H. Roles of inducible nitric oxide synthase in the development and healing of experimentally induced gastric ulcers. Int J Exp Pathol. 2003;84:213-220. [PubMed] [DOI] |
| 25. | Tatemichi M, Ogura T, Sakurazawa N, Nagata H, Sugita M, Esumi H. Inducible nitric oxide synthase activity induced by sodium chloride solution prolongs luminal pH elevation in rat and mouse stomachs. J Gastroenterol Hepatol. 2003;18:1039-1046. [PubMed] [DOI] |
| 27. | Hasumi K, Tanaka K, Saitoh S, Takagi A, Miwa T, Mine T, Koga Y. Roles of tumor necrosis factor-alpha-receptor type 1 and Fas in the Helicobacter pylori-induced apoptosis of gastric epithelial cells. J Gastroenterol Hepatol. 2002;17:651-658. [PubMed] [DOI] |
| 28. | Souza MH, Lemos HP, Oliveira RB, Cunha FQ. Gastric damage and granulocyte infiltration induced by indomethacin in tumour necrosis factor receptor 1 (TNF-R1) or inducible nitric oxide synthase (iNOS) deficient mice. Gut. 2004;53:791-796. [PubMed] [DOI] |
| 29. | Helmer KS, Cui Y, Chang L, Dewan A, Mercer DW. Effects of ketamine/xylazine on expression of tumor necrosis factor-alpha, inducible nitric oxide synthase, and cyclo-oxygenase-2 in rat gastric mucosa during endotoxemia. Shock. 2003;20:63-69. [PubMed] [DOI] |
| 30. | Sánchez S, Martín MJ, Ortiz P, Motilva V, Herrerías JM, Alarcón de la Lastra C. Role of prostaglandins and nitric oxide in gastric damage induced by metamizol in rats. Inflamm Res. 2002;51:385-392. [PubMed] [DOI] |