修回日期: 2004-05-09
接受日期: 2002-05-11
在线出版日期: 2004-09-15
目的: 克隆人类抗凋亡基因survivin(SVV), 实现SVV基因在大肠杆菌中的高效表达.
方法: 提取胃癌细胞系SGC-7901总RNA, RT-PCR扩增人SVV全长cDNA, 克隆于载体pGEM-T, 酶切、测序鉴定. 将人类SVV基因全长cDNA克隆入原核表达载体pRSET, 实现该基因在大肠杆菌BL21(DE3)中的高效表达.
结果: 得到人SVV基因全长cDNA, 经测序与SVV基因的已知序列相同, 原核表达所获融合蛋白经SDS-PAGE电泳与已知SVV基因的蛋白质表达产物大小相近.
结论: 克隆出人SVV基因的全长cDNA, 并克隆入原核表达载体pRSET, 并在大肠杆菌BL21(DE3)中高效表达.
引文著录: 杨欣艳, 王孟薇, 王刚石, 尤纬缔. 人类抗凋亡基因survivin的克隆及其原核表达. 世界华人消化杂志 2004; 12(9): 2123-2126
Revised: May 9, 2004
Accepted: May 11, 2002
Published online: September 15, 2004
AIM: To clone and express human antiapoptosis gene-survivin (SVV) in Escherchia coli.
METHODS: The SVV cDNA was obtained by using RT-PCR method with total RNA extracted from the human gastric cancer cell line, SGC7901. Then it was cloned into the pGEM-T easy vector, and subcloned into expression vector pRSET. After proved to be correct by sequencing, recombinant expression plasmid pRSET-SVV was transformed into E.coli BL21 (DE3). The fusion protein was produced by IPTG induction.
RESULTS: The SVV cDNA was obtained and its sequence was proved to be correct by sequencing identification, a new anticipated Mr 19 500-protein band appeared on SDS-PAGE gel induced by IPTG.
CONCLUSION: Human SVV cDNA is cloned and highly expressed in E.coli. This is important for studying its functions in the carcinogenesis and progress of neoplasm.
- Citation: Yang XY, Wang MW, Wang GS, You WD. Cloning and expression of human anti-apoptosis gene survivin in Escherchia coli. Shijie Huaren Xiaohua Zazhi 2004; 12(9): 2123-2126
- URL: https://www.wjgnet.com/1009-3079/full/v12/i9/2123.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v12.i9.2123
Survivin(SVV)是凋亡抑制蛋白(IAP)基因家族的新成员, SVV基因在人类大多数肿瘤组织中均呈异常高表达[1]. 该基因不仅与肿瘤的发生及进展有关, 还与肿瘤的预后和复发有关. 目前, 多采用自Hela细胞中克隆获得的SVV cDNA研究其在多种肿瘤中的表达, 研究其抗凋亡功能与肿瘤的相关性, 但除了其抗凋亡效应外, SVV在人类恶性肿瘤发生、发展中的生物学功能尚未阐明. 国内外也尚未见有自胃癌细胞中克隆SVV基因的报道. 为研究SVV基因在胃癌发生、发展中的生物学作用, 进一步了解其功能, 我们拟直接从胃癌细胞中克隆SVV基因, 并对其原核表达进行研究.
胃癌细胞株SGC7901购自中国科学院上海细胞所, 大肠杆菌JM109感受态细胞、pGEM-T vector system、T4DNA连接酶购自Promega公司; Trizol试剂、SuperScriptTM逆转录试剂盒、原核表达载体pRSET, EcoRⅠ及PCR Taq酶为Gibco 公司产品. 引物由上海博亚生物公司合成. 设计扩增survivin基因的引物: 引物设计应用C-Primer软件. SVV引物: SVV-P1-primer: 5'-GGGACCCGTTGGCAGAG-3'; SVV-P2-primer : 5'-AAAATGAGCCCCCAAAAAAGA-3', 预计扩增产物长度727 bp; 巢式引物: nest-SVV-P1-primer : 5'-TGCCCCACTGAGAACGAGCC-3', nest-SVV-P2-primer: 5'-GCCACTGTTACCAGCAGCACCC-3', 预计扩增产物长度500bp. 原核表达引物: SVV-P1- NdeⅠ: 5'-CATATGGGACCCGTTGGCAGAG-3' SVV-P2- BamHⅠ:5'-GGATCCAAAATGAGCCCCCAAAAAAGA-3'(产物预计长度739 bp).
胃癌细胞系SGC-7901在含100 mL/L小牛血清的DMEM培养基中培养(50 mL/L CO2, 37 ℃)至对数生长期, 取约1×106个细胞, 用Trizol试剂按产品说明提取总RNA, 甲醛变性琼脂糖凝胶电泳检查RNA的完整性, 紫外分光光度法分析RNA的纯度及含量. 逆转录反应、PCR扩增: 取1 mg总RNA, 按照试剂盒说明进行逆转录反应. 以逆转录产物为模板, 以SVV引物进行PCR反应, 反应条件为: 94 ℃, 2 min; 94 ℃, 30 s; 50 ℃, 30 s; 72 ℃, 1 min; 35个循环, 最后72 ℃延伸7 min. 以PCR产物为模板, 以SVV巢式引物进行巢式PCR鉴定, 反应条件为: 95 ℃, 2 min; 94 ℃, 30 s; 60 ℃, 30 s; 72 ℃, 1 min; 25个循环, 最后72 ℃延伸7 min. PCR产物在10 g/L琼脂糖凝胶上电泳鉴定. PCR产物经10 g/L 低熔点琼脂糖凝胶电泳, 在长波紫外灯下(3 650 nm)切胶回收, 酚/氯仿抽提、纯化, 连接于pGEM-T easy载体, 转化大肠杆菌JM109感受态细胞, 采用蓝白斑筛选试验筛选阳性克隆, 提取质粒, EcoR I酶切分析鉴定转化重组子. 阳性克隆的DNA序列测定由上海博亚生物公司完成. SVV基因的原核表达: 以阳性SVV克隆(命名为S4A)质粒为模板, 以原核表达引物PCR扩增引入NdeⅠ和BamHⅠ限制性内切酶识别位点的片段(命名为S6), 将该PCR产物纯化后与pRSET载体分别用NdeⅠ和BamHⅠ双酶切, 低熔点琼脂糖回收S6片段和pRSET载体, 鉴定后连接, 组成新的重组质粒pRSET- S6, 测序鉴定. 挑取含重组质粒pRSET- S6的单菌落于2 mL含氨苄青霉素的SOB培养基中, 37 ℃培养过夜. 取0.2 mL培养过夜的培养基至50 mL新鲜SOB培养基中, 37 ℃剧烈振摇至A600 = 0.3, 取1 mL菌液, 10 000 g离心5 min收获菌体, -20 ℃保存, (此为未诱导的对照样品, 命名为0 h), 之后加入IPTG至终浓度1 mmol/L, 再37 ℃振摇4、6、8 h, 分别取菌液1 mL, 离心后收集菌体, -20 ℃保存. 用上样缓冲液重悬菌体, 经液氮-42 ℃反复冻融3-4次, 分别取样品30 mL与蛋白质标准品20 mL一起置沸水中变性5 min, SDS-PAGE凝胶电泳, 考马斯亮蓝染色, 分析外源蛋白的表达情况.
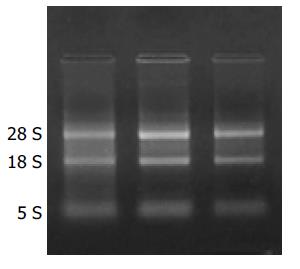
从胃癌细胞系SGC-7901提取的总RNA经甲醛变性琼脂糖凝胶电泳, 可见清晰的28 s, 18 s和5 s条带, 而且28 s的亮度大约是18 s的2倍, 有较好的完整性. A260/A280 = 1.64, 说明RNA有较高的纯度(图1).
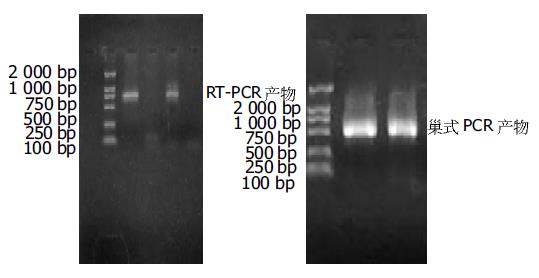
将RT-PCR与巢式PCR产物行10 g/L琼脂糖凝胶电泳, 可见扩增片段大小分别约727 bp, 500 bp(图2), 与扩增产物的预计长度相符.
SVV基因与载体连接并转化后, 从培养板上随机挑选白色克隆, 摇菌扩增提取质粒, 经EcoRⅠ酶切鉴定, 含有插入片段, 将此阳性克隆命名为S4A. 将S4A以SP6, T7引物进行序列测定. 测序结果显示S4A的序列与已知的SVV序列完全一致, 黑色斜体部分为SVV基因PCR扩增之上游引物.
ACGTCGCATGCTCCCGGCCGCCATGGCGGCCGCGGGAATTCGATTGGGACCCGTTGGCAGAGGTGGCG
GCGGCGGCATGGGTGCCCCGACGTTGCCCCCTGCCTGGCAGCCCTTTCTCAAGGACCACCGCATCTCTA
CATTCAAGAACTGGCCCTTCTTGGAGGGCTGCGCCTGCACCCGGGAGCGGATGGCCGAGGCTGGCTTCA
TCCACTGCCCCACTGAGAACGAGCCAGACTTGGCCCAGTGTTTCTTCTGCTTCAAGGAGCTGGAAGGCT
GGGAGCCAGATGACGACCCCATAGAGGAACATAAAAAGCATTCGTCCGGTTGCGCTTTCCTTTCTGTCA
AGAAGCAGTTTGAAGAATTAACCCTTGGTGAATTTTTGAAACTGGACAGAGAAAGAGCCAAGAACAAAA
TTGCAAAGGAAACCAACAATAAGAAGAAAGAATTTGAGGAAACTGCGAAGAAAGTGCGCCGTGCCATCG
AGCAGCTGGCTGCCATGGATTGAGGCCTCTGGCCGGAGCTGCCTGGTCCCAGAGTGGCTGCACCACTTC
CAGGGTTTATTCCCTGGTGCCACCAGCCTTCCTGTGGGCCCCTTAGCAATGTCTTAGAAAAGGAGATCA
ACATTTTCAAATTAAGATGTTTCAACTGTGCTTCTTGTTTTTGTCTTTG
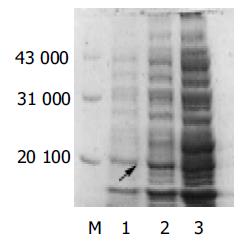
表达的融合蛋白Mr 19 500(融合蛋白部分约3 000)(图3), 所表达的融合蛋白分子大小与理论值相符, pRSET-SVV转化之大肠杆菌经IPTG诱导后, 目的蛋白的表达明显增多, 而未经诱导者则几无该目的蛋白的表达.
抗凋亡蛋白(IAP)基因家族是普遍表达的凋亡抑制基因家族. SVV基因是IAP家族中的一个新成员. 人类SVV基因定位于染色体17q25, mRNA全长1.9 kb, 该基因编码一个142个氨基酸、Mr 16 500的蛋白质. SVV基因的结构与该家族的其他成员不同, 即仅含有单一的BIR功能区, 没有环指结构[1]. 晶体结构分析揭示, 人类SVV包括蝶形领结状的二聚体结构和2个独特的a螺旋侧链, C端螺旋包含1个疏水区, 该结构提示SVV蛋白存在蛋白-蛋白相互作用的功能[2-3]. SVV基因不仅具有抗凋亡功能, 且与组织分化有关[1,4]. 研究表明, SVV基因在大多数肿瘤细胞系中均有表达, 在大多数人类肿瘤组织如神经母细胞瘤、肺癌、妇科肿瘤、肝癌、胃肠道肿瘤等均有高表达[5-16]; SVV基因不仅与肿瘤的发生及进展有关, 还与肿瘤的预后和复发有关[5-6,17-25]. SVV在多种肿瘤表达的普遍性, 使得应用靶向SVV的阻断性抗体免疫治疗或基因治疗促进肿瘤细胞凋亡的抗肿瘤疗法成为可能[26-29].
目前, 多采用自Hela细胞中克隆获得的SVVcDNA研究其在多种肿瘤中的表达, 研究其抗凋亡功能与肿瘤的相关性, 但除了其抗凋亡效应外, SVV在人类恶性肿瘤中发生、发展中的生物学功能尚未阐明. 国内外也尚未见有自胃癌细胞中克隆SVV基因的报道. 基于上述, 我们从胃癌细胞SGC7901中成功克隆SVV基因, 并对其原核表达进行了研究, 为以后的工作准备了条件. SVV基因编码蛋白质的碱基(CDS)为1 619 bp(GENEBANK登陆号NM 001168), 我们选择包含有编码蛋白质开放阅读框架的片段19-734 bp作为扩增的靶片段, 扩增相应的片段. 经酶切分析和靶片段的克隆测序证实克隆片段序列的正确性, 并将其与原核表达载体pRSET相连接, 阳性重组子pRSET-SVV经测序证实克隆片段与载体连接正确, 且克隆片段具有完整的开放阅读框架. 将该阳性重组子转染大肠杆菌BL21(DE3)细胞, SDS-PAGE凝胶电泳和考马斯亮蓝染色后可见转化的细胞中有相应的蛋白表达. SVV基因的氨基酸序列分析表明, SVV蛋白在Thr21, Ser88, Thr127包含3个蛋白激酶C磷酸化位点, 在Thr48和 Thr97处有2个酪蛋白激酶2磷酸化位点, 在Ser81有1个蛋白激酶A磷酸化位点, 上述结构对于蛋白质的磷酸化作用或对于凋亡的潜在调节作用尚未阐明[30].
我们成功地进行了SVV基因的原核表达, 为以后相关抗体的制备提供了条件, 目前正在进行包括SVV在内的抗体库筛选工作, 这将对肿瘤的诊断、治疗具有重要的价值. 国内也有SVV mAb制备的相关研究. 再者, 通过对SVV基因结构的分析显示, SVV蛋白存在蛋白-蛋白相互作用的可能[2-3]; Shako et al[31]发现, 泛激素-蛋白酶通路以细胞周期依赖的形式调节SVV蛋白质的降解和结构变化, 明显减低SVV蛋白质的稳定性, 说明SVV蛋白的表达存在翻译水平的调节. 我们构建SVV基因原核表达载体对于以后详细的研究SVV蛋白的功能和其相关蛋白质之间的相互作用提供了前提条件.
| 1. | Ambrosini G, Adida C, Altieri DC. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med. 1997;3:917-921. [PubMed] [DOI] |
| 2. | Verdecia MA, Huang H, Dutil E, Kaiser DA, Hunter T, Noel JP. Structure of the human anti-apoptotic protein survivin reveals a dimeric arrangement. Nat Struct Biol. 2000;7:602-608. [PubMed] [DOI] |
| 3. | Chantalat L, Skoufias DA, Kleman JP, Jung B, Dideberg O, Margolis RL. Crystal structure of human survivin reveals a bow tie-shaped dimer with two unusual alpha-helical extensions. Mol Cell. 2000;6:183-189. [PubMed] [DOI] |
| 4. | Zhu H, Liu S, Zhou C, Zhou X, Zhang F, Jin L, Xu N. [Anti-apoptosis gene survivin promotes cell growth and transformation]. Zhonghua Yixue Zazhi. 2002;82:338-340. [PubMed] |
| 5. | Sarela AI, Macadam RC, Farmery SM, Markham AF, Guillou PJ. Expression of the antiapoptosis gene, survivin, predicts death from recurrent colorectal carcinoma. Gut. 2000;46:645-650. [PubMed] [DOI] |
| 6. | Islam A, Kageyama H, Takada N, Kawamoto T, Takayasu H, Isogai E, Ohira M, Hashizume K, Kobayashi H, Kaneko Y. High expression of Survivin, mapped to 17q25, is significantly associated with poor prognostic factors and promotes cell survival in human neuroblastoma. Oncogene. 2000;19:617-623. [PubMed] [DOI] |
| 7. | Takai N, Miyazaki T, Nishida M, Nasu K, Miyakawa I. Expression of survivin is associated with malignant potential in epithelial ovarian carcinoma. Int J Mol Med. 2002;10:211-216. [PubMed] [DOI] |
| 8. | Frost M, Jarboe EA, Orlicky D, Gianani R, Thompson LC, Enomoto T, Shroyer KR. Immunohistochemical localization of survivin in benign cervical mucosa, cervical dysplasia, and invasive squamous cell carcinoma. Am J Clin Pathol. 2002;117:738-744. [PubMed] [DOI] |
| 9. | Sarela AI, Verbeke CS, Ramsdale J, Davies CL, Markham AF, Guillou PJ. Expression of survivin, a novel inhibitor of apoptosis and cell cycle regulatory protein, in pancreatic adenocarcinoma. Br J Cancer. 2002;86:886-892. [PubMed] [DOI] |
| 10. | McEleny KR, Watson RW, Coffey RN, O'Neill AJ, Fitzpatrick JM. Inhibitors of apoptosis proteins in prostate cancer cell lines. Prostate. 2002;51:133-140. [PubMed] [DOI] |
| 11. | Mori A, Wada H, Nishimura Y, Okamoto T, Takemoto Y, Kakishita E. Expression of the antiapoptosis gene survivin in human leukemia. Int J Hematol. 2002;75:161-165. [PubMed] [DOI] |
| 12. | Wang X, Chen S, Zhang Z. [Expression of surviving gene and its relationship with expression of P53, c-myc, k-ras proteins in non-small-cell lung cancer]. Zhonghua Jiehe He Huxi Zazhi. 2001;24:371-374. [PubMed] |
| 13. | Azuhata T, Scott D, Takamizawa S, Wen J, Davidoff A, Fukuzawa M, Sandler A. The inhibitor of apoptosis protein survivin is associated with high-risk behavior of neuroblastoma. J Pediatr Surg. 2001;36:1785-1791. [PubMed] [DOI] |
| 14. | Satoh K, Kaneko K, Hirota M, Masamune A, Satoh A, Shimosegawa T. Expression of survivin is correlated with cancer cell apoptosis and is involved in the development of human pancreatic duct cell tumors. Cancer. 2001;92:271-278. [PubMed] [DOI] |
| 15. | Kawasaki H, Toyoda M, Shinohara H, Okuda J, Watanabe I, Yamamoto T, Tanaka K, Tenjo T, Tanigawa N. Expression of survivin correlates with apoptosis, proliferation, and angiogenesis during human colorectal tumorigenesis. Cancer. 2001;91:2026-2032. [PubMed] [DOI] |
| 16. | Granziero L, Ghia P, Circosta P, Gottardi D, Strola G, Geuna M, Montagna L, Piccoli P, Chilosi M, Caligaris-Cappio F. Survivin is expressed on CD40 stimulation and interfaces proliferation and apoptosis in B-cell chronic lymphocytic leukemia. Blood. 2001;97:2777-2783. [PubMed] [DOI] |
| 17. | Sandler A, Scott D, Azuhata T, Takamizawa S, O'Dorisio S. The survivin:Fas ratio is predictive of recurrent disease in neuroblastoma. J Pediatr Surg. 2002;37:507-511. [PubMed] [DOI] |
| 18. | Ikeguchi M, Ueda T, Sakatani T, Hirooka Y, Kaibara N. Expression of survivin messenger RNA correlates with poor prognosis in patients with hepatocellular carcinoma. Diagn Mol Pathol. 2002;11:33-40. [PubMed] [DOI] |
| 19. | Chakravarti A, Noll E, Black PM, Finkelstein DF, Finkelstein DM, Dyson NJ, Loeffler JS. Quantitatively determined survivin expression levels are of prognostic value in human gliomas. J Clin Oncol. 2002;20:1063-1068. [PubMed] [DOI] |
| 20. | Kamihira S, Yamada Y, Hirakata Y, Tomonaga M, Sugahara K, Hayashi T, Dateki N, Harasawa H, Nakayama K. Aberrant expression of caspase cascade regulatory genes in adult T-cell leukaemia: survivin is an important determinant for prognosis. Br J Haematol. 2001;114:63-69. [PubMed] [DOI] |
| 21. | Lo Muzio L, Staibano S, Pannone G, Mignogna MD, Mariggiò A, Salvatore G, Chieffi P, Tramontano D, De Rosa G, Altieri DC. Expression of the apoptosis inhibitor survivin in aggressive squamous cell carcinoma. Exp Mol Pathol. 2001;70:249-254. [PubMed] [DOI] |
| 22. | Sarela AI, Scott N, Ramsdale J, Markham AF, Guillou PJ. Immunohistochemical detection of the anti-apoptosis protein, survivin, predicts survival after curative resection of stage II colorectal carcinomas. Ann Surg Oncol. 2001;8:305-310. [PubMed] [DOI] |
| 23. | Kato J, Kuwabara Y, Mitani M, Shinoda N, Sato A, Toyama T, Mitsui A, Nishiwaki T, Moriyama S, Kudo J. Expression of survivin in esophageal cancer: correlation with the prognosis and response to chemotherapy. Int J Cancer. 2001;95:92-95. [PubMed] [DOI] |
| 24. | Okada E, Murai Y, Matsui K, Isizawa S, Cheng C, Masuda M, Takano Y. Survivin expression in tumor cell nuclei is predictive of a favorable prognosis in gastric cancer patients. Cancer Lett. 2001;163:109-116. [PubMed] [DOI] |
| 25. | Adida C, Haioun C, Gaulard P, Lepage E, Morel P, Briere J, Dombret H, Reyes F, Diebold J, Gisselbrecht C. Prognostic significance of survivin expression in diffuse large B-cell lymphomas. Blood. 2000;96:1921-1925. [PubMed] |
| 26. | Kanwar JR, Shen WP, Kanwar RK, Berg RW, Krissansen GW. Effects of survivin antagonists on growth of established tumors and B7-1 immunogene therapy. J Natl Cancer Inst. 2001;93:1541-1552. [PubMed] [DOI] |
| 27. | Mesri M, Wall NR, Li J, Kim RW, Altieri DC. Cancer gene therapy using a survivin mutant adenovirus. J Clin Invest. 2001;108:981-990. [PubMed] [DOI] |
| 28. | Grossman D, Kim PJ, Schechner JS, Altieri DC. Inhibition of melanoma tumor growth in vivo by survivin targeting. Proc Natl Acad Sci USA. 2001;98:635-640. [PubMed] [DOI] |
| 29. | Schmitz M, Diestelkoetter P, Weigle B, Schmachtenberg F, Stevanovic S, Ockert D, Rammensee HG, Rieber EP. Generation of survivin-specific CD8+ T effector cells by dendritic cells pulsed with protein or selected peptides. Cancer Res. 2000;60:4845-4849. [PubMed] |
| 30. | Altieri DC, Marchisio PC. Survivin apoptosis: an interloper between cell death and cell proliferation in cancer. Lab Invest. 1999;79:1327-1333. [PubMed] |
| 31. | Zhao J, Tenev T, Martins LM, Downward J, Lemoine NR. The ubiquitin-proteasome pathway regulates survivin degradation in a cell cycle-dependent manner. J Cell Sci. 2000;113 Pt 23:4363-4371. [PubMed] |