修回日期: 2004-06-09
接受日期: 2004-06-17
在线出版日期: 2004-09-15
目的: 探讨EBV相关胃癌(EBV associated gastric carcinoma, EBVaGC)病毒基因表达、Bcl-2蛋白表达与细胞凋亡的相互关系, 明确其在EBVaGC发生发展中的作用.
方法: 选取EBVaGC和相应癌旁组织13例, 与之匹配的EBVnGC 45例, 采用TUNEL法及免疫组化技术检测其细胞凋亡指数(AI)和bcl-2蛋白的表达; RT-PCR-Southern杂交技术检测EBV相关基因表达.
结果: EBVaGC、EBVnGC及EBVaGC癌旁组织中AI分别为0.97±0.41, 2.03±0.60和3.25±0.46, 统计学分析表明:EBVaGC和EBVnGC以及EBVaGC癌组织和相应癌旁组织细胞凋亡指数均有显著性差异(t = 5.9795, P = 0; t = 13.2 229, P = 0). EBVaGC 组和EBVnGC组Bcl-2阳性率分别为 53.8%和48.9%, 两组间无显著性差异(χ2 = 0.0991 , P = 0.7 529). EBVaGC 13例EBNA1 mRNA均阳性, 而EBNA2和LMP1 mRNA均阴性; EBV早期基因BARF1表达阳性6例, BHRF1表达阳性2例.
结论: EBVaGC 癌组织Bcl-2表达与EBV感染无明显相关性, EBV感染抑制细胞凋亡不是上调Bcl-2表达而实现, EBV早期基因BARF1和BHRF1可通过抑制细胞凋亡和细胞转化作用参与EBVaGC的发生.
引文著录: 王笑峰, 罗兵, 王云, 阎丽平, 黄葆华, 赵鹏. EB病毒相关胃癌细胞凋亡与Bcl-2的表达. 世界华人消化杂志 2004; 12(9): 2028-2032
Revised: June 9, 2004
Accepted: June 17, 2004
Published online: September 15, 2004
AIM: To understand the relationship between Epstein-Barr virus related genes expression, Bcl-2 expression and apoptosis in EBV-associated gastric carcinoma (EBVaGC) and their roles in the oncogenesis and development of gastric carcinoma.
METHODS: The apoptotic index (AI) and the expression of Bcl-2 protein were detected by terminal deoxynucleotidy1 transferase (TdT)-mediated dUTP-biotin nick end labeling (TUNEL) and immunohistochemistry, respectively, in 13 cases of EBVaGC and 45 EBVnGC. The expression of EBV related genes was tested by RT-PCR and Southern blotting.
RESULTS: AI of EBVaGC, EBVnGC and the corresponding adjacent tissues of EBVaGC were 0.97±0.41, 2.03±0.60 and 3.25±0.46, respectively. AI of EBVaGC was significantly lower than that of EBVnGC (t = 5.9 795, P = 0) and corresponding adjacent tissues of EBVaGC (t = 13.2 229, P = 0). The expression of Bcl-2 protein was detectded in 7 of 13 (53.8%) EBVaGC and in 22 of 45 (48.9%) EBVnGC. The difference between the two groups was not significant(χ2 = 0.0 991, P = 0.7 529). EBNA1 mRNA was detected in all of 13 EBVaGC, while both EBNA2 and LMP1 mRNA were not detected in the cases. Of the 13 EBV-positive samples,6 exhibited BARF1 transcripts and 2 exhibited BHRF1 transcripts.
CONCLUSION: Bcl-2 expression does not correlate with the presence of EBV in EBVaGC. EBV infection can inhibit cell apoptosis not by Bcl-2 expression. Early genes BARF1 and BHRF1 might play an important role in the development and progression of gastric carcinomas by the mechanisms of immortalizing epithelial cells and inhibiting cell apoptosis.
- Citation: Wang XF, Luo B, Wang Y, Yan LP, Huang BH, Zhao P. Apoptosis and expression of Bcl-2 in Epstein-Barr virus-associated gastric carcinoma. Shijie Huaren Xiaohua Zazhi 2004; 12(9): 2028-2032
- URL: https://www.wjgnet.com/1009-3079/full/v12/i9/2028.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v12.i9.2028
Epstein-Barr 病毒(EBV)与胃癌的相关性已得到证实[1-6], 但EBV致癌机制尚未明确, EBV感染与细胞凋亡和增生相关基因的相互作用成为EBV致癌机制研究的热点. 我们采用末端脱氧核苷酸移换酶(TdT)介导的dUTP缺口原位末端标记法(TUNEL)与免疫组化技术分别检测13例EBVaGCs和相应癌旁组织以及与之相匹配的45例EBV阴性胃癌(EBV-negative gastric carcinoma, EBVnGC)组织中的细胞凋亡指数(apoptotic index, AI)和Bcl-2蛋白的表达, RT-PCR检测EBVaGC组织中病毒潜伏期基因EBNA1、EBNA2、LMP1和病毒早期基因BARF1和BHRF1的表达, 探讨EBVaGC中EBV相关基因表达与细胞凋亡和Bcl-2表达的关系及其在胃癌发生发展中的作用.
2001-01/2002-12胃癌手术切除的新鲜胃癌组织和相应癌旁组织(距离癌组织5 cm以上) 185例, 用PBS-40 g/L甲醛固定, 石蜡切片. 另取新鲜组织用酚、氯仿-异戊醇法常规提取组织DNA, TRIzol试剂提取细胞总RNA. 患者平均年龄58(31-81岁), 男48例, 女10例, 全部病例均经病理诊断证实.
参照文献[7]设计扩增该区域的特异性引物, 引物序列和PCR-Southern用探针序列(见表1).
| 转录产物 | 引物序列(5'→3') | 产物长度(bp) | |
| BamHI-W: | 5'primer | CCAGACAGCAGCCAATTGTC | 129 |
| 3'primer | GGTAGAAGACCCCCTCTTAC | ||
| probe | CCCTGGTATAAAGTGGTCCTGCAGCTATTTCTGGTCGCATC | ||
| EBER1: | antisense probe | AGACACCGTCCTCACCCACCCGGGACTTGTA | |
| sense probe | TCTGTGGCAGGAGTGGTGGGCCCTGAACAT |
选取PCR-Southern阳性标本进行原位杂交(地高辛标记检测试剂盒)检测EBV编码小RNA(EBER1)的转录, 以确证EBV相关胃癌, 特异性寡核苷酸探针序列(见表1). Sense探针用于证实杂交反应的特异性. EBV潜伏期基因EBNA1, EBNA2, LMP1和EBV增生期基因特异性引物和探针均参照文献[8-10]设计, 由北京赛百盛公司合成, 引物和探针序列以及PCR产物长度(见表2). 应用Roche公司提供的Dig Oligonucleotide 3'-end Labeling Kit对寡核苷酸探针进行标记, 具体步骤按试剂盒要求的标准条件进行.
| 转录产物 | 引物序列(5'→3') | 产物长度(bp) | |
| EBNA1: | 5'primer | GATGAGCGTTTGGGAGAGCTGATTCTGCA | 273 |
| 3' primer | TCCTCGTCCATGGTTATCAC | ||
| probe | AGACCTGGGAGCAGATTCAC | ||
| EBNA2: | 5' primer | GCTGCTACGCATTAGAGACC | 339 |
| 3' primer | TCCTGGTAGGGATTCGAGGG | ||
| probe | CAGCACTGGCGTGTGACGTGGTGTAAGTT | ||
| LMP1: | 5'primer | TCCTCCTCTTGGCGCTACTG | 490 |
| 3'primer | TCATCACTGTGTCGTTGTCC | ||
| probe | GAACAGCACAATTCCAAGGAACAATGCCTG | ||
| BARF1: | 5'primer | GGCTGTCACCGCTTTCTTGG | 203 |
| 3'primer | AGGTGTTGGCACTTCTGTGG | ||
| probe | CTGGTTTAAACTGGGCCCAGGAGAGGAGCA | ||
| BHRF1: | 5'primer | GTCAAGGTTTCGTCTGTGTG | 211 |
| 3'primer | TTCTCTTGCTGCTAGCTCCA | ||
| probe | ATGCACACGACTGTCCCGTATACAC |
1.2.1 RT-PCR 检测EBV相关基因表达: 取1 mg EBVaGC组织总RNA, 参照逆转录试剂盒(Promega, USA)要求的标准条件合成cDNA用作PCR反应模板. PCR反应体系为30 mL, 包括10×buffer 3 mL, MgCl2 1.5 mmol/L, dNTP 0.1 mmol/L, 上下游引物各0.5 mmmol/L, Taq DNA聚合酶1.0U, cDNA聚合酶1U, cDNA模板3 mL, 同时设立阳性对照和阴性对照, 阳性对照为EBV阳性的LCL细胞, 阴性对照为EBV阴性Ramos细胞系. 循环参数为94 ℃预变性5 min; 然后94 ℃ 45 s, 58 ℃ 45 s, 72 ℃ 1 min, 扩增35个循环; 最后72 ℃延伸10 min. PCR扩增产物于含溴化乙锭(0.5 mg/L)的20 g/L琼脂糖凝胶电泳后, 用碱变性法将电泳结果转印至Hybond-N+尼龙膜(amersham pharmacia biotec, IRELAND). 与地高辛标记探针进行杂交, 加碱性磷酸酶标记抗地高辛抗体(1:5 000)作用30 min, 用CSPD化学发光检测杂交信号, 自显影观察结果.
1.2.2 免疫组化检测Bcl-2蛋白表达: 免疫组化抗原热修复技术检测Bcl-2蛋白表达, 抗原修复液为枸橼酸缓冲液(pH6.0), 一抗为鼠抗人Bcl-2蛋白mAb SC-7382(santa cruz biotechnology, Inc USA), SP通用型染色试剂盒和DAB显色试剂盒购于北京中山生物技术有限公司. 用PBS代替一抗作阴性对照; 已知Bcl-2阳性的扁桃腺组织为阳性对照. Bcl-2蛋白免疫组化染色阳性信号出现在胞质内, 呈棕褐色颗粒, 参考文献确定Bcl-2蛋白染色结果判断标准, 10×40倍镜下随机选取5个视野, 阳性细胞数量为0-5%视为阴性(-), >5%视为阳性(+).
1.2.3 TUNEL法检测细胞凋亡: 应用TUNEL原位细胞凋亡检测试剂盒(武汉博士德生物工程有限公司), 严格按照试剂盒说明对EBVaGC和相应癌旁组织石蜡切片进行检测, DAB显色后观察, 细胞核中出现棕黄色颗粒者为阳性细胞, 即凋亡细胞. 10×40倍下随机选取5个视野, 计数1 000个癌细胞或癌旁正常细胞中凋亡细胞的百分数作为AI指数.
统计学处理 癌组织和癌旁组织AI指数采用配对四格表χ2检验进行统计分析, EBVaGC和EBVnGC的Bcl-2阳性表达及AI指数采用四格表χ2检验进行统计分析.
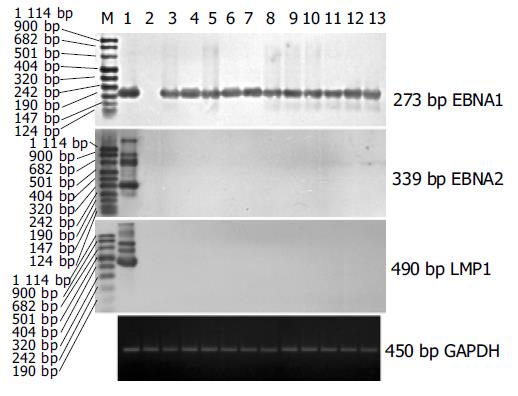
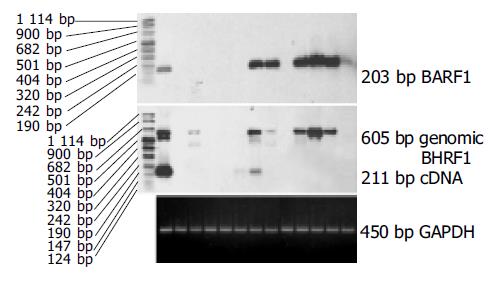
PCR-Southern杂交检测185例胃癌组织中EBV DNA阳性标本经原位杂交证实有13例EBVaGC, 185例相应癌旁组织EBV阴性. 13例EBVaGC组织中均检测到内参照基因GAPDH的表达, 表明cDNA的完整性和PCR反应成功. 13例EBVaGC组织中均检测到EBNA1 mRNA, 而EBNA2和LMP1 mRNA均为阴性(图1) . 13例EBVaGC组织中有6例检测到EBV早期基因BARF1的表达, 2例检测到EBV早期基因BHRF1的表达, RT-PCR-Southern检测结果(见图2).
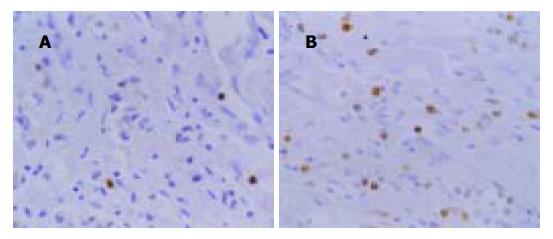
TUNEL法检测EBVaGC和EBVnGC细胞凋亡结果(见图3A, B), EBVaGC, EBVnGC及EBVaGC癌旁组织中AI分别为0.97±0.41, 2.03±0.60和3.25±0.46. 统计学分析表明, EBVaGC与EBVnGC比较AI值的差别有显著性(t = 5.9 795, P = 0), EBVaGC癌组织和相应癌旁组织AI值的差别亦有显著性(t = 13.2 229, P = 0).
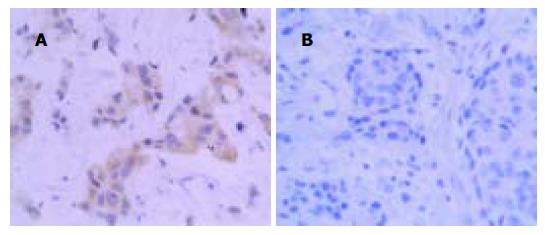
胃癌组织EBVaGC和EBVnGC免疫组化检测结果(见图4A, B). 统计学分析结果表明, EBVaGC 组Bcl-2阳性率为53.8%(7/13), EBVnGC Bcl-2阳性率为48.9(22/45), 两组之间无显著性差异(χ2 = 0.0 991, P = 0.7 529).
Bcl-2是目前已知抑制细胞凋亡最重要的一种基因, 其编码蛋白是抑制细胞程序化死亡的关键因子, Bcl-2的高表达能使细胞凋亡减少, 存活细胞数量增多而诱使肿瘤发生[11-14]. 有关EBVaGC中EBV感染与细胞凋亡、Bcl-2蛋白表达的关系, 目前尚无定论. Kume et al[15]和Ohfuji et al[16]发现EBV相关胃癌中Bcl-2表达率高且细胞凋亡率低, 因而认为Bcl-2蛋白是EBV相关胃癌细胞凋亡的主要抑制因素, EBV感染可能诱导Bcl-2高表达. Ishii et al[17]应用免疫组化技术检测Bcl-2等癌基因的表达, 结果显示EBVaGC和EBVnGC癌组织中Bcl-2的表达率无明显差异. 本结果显示EBVaGC较EBVnGC细胞凋亡率明显降低, 但Bcl-2表达率均无显著性差异, 与Ishii et al的研究结果相似. 表明EBVaGC中EBV可能抑制细胞凋亡, 但不影响Bcl-2的表达, Bcl-2蛋白异常表达不是 EBVaGC细胞凋亡发生的主要抑制因素.
EBV裂解感染和潜伏性感染与细胞凋亡的调节机制具有复杂多样的关系, EBV复制过程中各阶段表达的病毒蛋白对细胞凋亡也有不同的调节作用. EBV潜伏膜蛋白LMP1是主要病毒癌蛋白之一, 可通过上调Bcl-2原癌基因的表达抑制B淋巴细胞的凋亡. LMP1还可通过激活A20基因阻断p53介导的细胞凋亡, EBNA2可加强LMP1对Bcl-2基因表达的诱导作用, LMP1上调Bcl-2的表达, 抑制感染B淋巴细胞的凋亡, 因而可维持EBV 在B淋巴细胞中持续存在[18-21]. EBV早期基因BHRF1与Bcl-2的基因序列具有高度的同源性, 实验表明BHRF1除具有与Bcl-2相似的抑制B淋巴细胞和上皮细胞凋亡的作用, 又具有Bcl-2所没有的促进细胞生长和转化的作用[22-24]. Horner et al[25]将BHRF1基因导入人鳞癌细胞株SCC12F, 发现BHRF1的表达可延迟细胞的终极分化, 增强对DNA损伤药物的耐受力以及血清缺乏情况下细胞的生存能力, 由于上皮细胞的分化是一个细胞凋亡过程, 因而这一资料表明, BHRF1通过抑制细胞凋亡而阻止细胞分化.
体外研究表明EBV潜在膜蛋白LMP1和EBNA2能诱导Bcl-2蛋白的表达, 但临床研究未能证实体内也存在这种机制[18-21,26-27].为探讨EBVaGC患者体内是否存在该作用机制, Gulley et al[26]对95例胃癌组织中Bcl-2表达和EBV感染进行检测分析, 结果表明Bcl-2蛋白约在1/3的胃癌组织中表达, Bcl-2的表达不依赖于EBV的存在, 而且没有被病毒LMP所上调. 本研究结果显示13例EBVaGC组织中均未检测到LMP1和EBNA2 mRNA, 支持Gulleyet al的研究结论. 即使在EBVnGC癌组织中Bcl-2也约有48.9%的表达, 与EBVaGC癌组织中Bcl-2蛋白阳性率比较无显著性差异, 提示EBVaGC组织中Bcl-2表达不是由LMP1诱导产生. 研究证实EBV另一早期基因BARF1可在体外转化原代猴肾上皮细胞和人上皮细胞, 若将BARF1基因导入鼠成纤维细胞系BALB/c3T3或EBV阴性的B细胞系Louckes, 则产生致瘤转化, 转化的鼠成纤维细胞接种至新生鼠体内, 可形成进行性表达BARF1的肿瘤[28-30]. Zur Hausen et al[10]利用RT-PCR和Southern杂交检测10例EBV阳性胃腺癌, 结果显示9例表达BARF1, 2例表达BHRF1, 而LMP1均为阴性. 本研究13例EBVaGCs中有6例BARF1 mRNA阳性, 2例 BHRF1 mRNA阳性, 支持Hausen et al的结果. 鉴于大多数研究表明EBVaGC LMP1表达缺失, 因此推测BARF1和BHRF1在EBVaGC中可能替代LMP1起到致癌作用.
总之, EBV感染与胃癌的发生有关, EBV及其编码蛋白通过不同的作用方式和途径抑制肿瘤细胞凋亡, 但抑制细胞凋亡的机制不是通过上调Bcl-2的表达而实现. EBVaGC组织中LMP1和EBNA2表达阴性, 与Bcl-2的表达和细胞凋亡的发生无明显相关性, EBV早期基因BARF1和BHRF1编码产物可通过转化细胞和抑制细胞凋亡, 在EBVaGC的发生中起重要作用.
| 1. | Wang Y, Luo B, Zhao P, Huang BH. [Expression of Epstein-Barr virus genes in EBV-associated gastric carcinoma]. Ai Zheng. 2004;23:782-787. [PubMed] |
| 2. | Zur Hausen A, van Rees BP, van Beek J, Craanen ME, Bloemena E, Offerhaus GJ, Meijer CJ, van den Brule AJ. Epstein-Barr virus in gastric carcinomas and gastric stump carcinomas: a late event in gastric carcinogenesis. J Clin Pathol. 2004;57:487-491. [PubMed] [DOI] |
| 3. | Imai S, Koizumi S, Sugiura M, Tokunaga M, Uemura Y, Yamamoto N, Tanaka S, Sato E, Osato T. Gastric carcinoma: monoclonal epithelial malignant cells expressing Epstein-Barr virus latent infection protein. Proc Natl Acad Sci USA. 1994;91:9131-9135. [PubMed] [DOI] |
| 4. | Luo B, Murakami M, Fukuda M, Fujioka A, Yanagihara K, Sairenji T. Characterization of Epstein-Barr virus infection in a human signet ring cell gastric carcinoma cell line, HSC-39. Microbes Infect. 2004;6:429-439. [PubMed] [DOI] |
| 5. | Lee MA, Hong YS, Kang JH, Lee KS, You JY, Lee KY, Park CH. Detection of Epstein-Barr virus by PCR and expression of LMP1, p53, CD44 in gastric cancer. Korean J Intern Med. 2004;19:43-47. [PubMed] [DOI] |
| 6. | Andal N, Shanthi P, Krishnan KB, Taralaxmi V. The Epstein Barr virus and gastric carcinoma. Indian J Pathol Microbiol. 2003;46:34-36. [PubMed] |
| 7. | 罗 兵, 村 上雅尚, 柳 原五吉, 西 连寺刚. EB病毒对人胃癌细胞系HSC-39感染的研究. 中华微生物学和免疫学杂志. 2002;22:379-384. |
| 8. | Oudejans JJ, van den Brule AJ, Jiwa NM, de Bruin PC, Ossenkoppele GJ, van der Valk P, Walboomers JM, Meijer CJ. BHRF1, the Epstein-Barr virus (EBV) homologue of the BCL-2 protooncogene, is transcribed in EBV-associated B-cell lymphomas and in reactive lymphocytes. Blood. 1995;86:1893-1902. [PubMed] |
| 9. | Sugiura M, Imai S, Tokunaga M, Koizumi S, Uchizawa M, Okamoto K, Osato T. Transcriptional analysis of Epstein-Barr virus gene expression in EBV-positive gastric carcinoma: unique viral latency in the tumour cells. Br J Cancer. 1996;74:625-631. [PubMed] [DOI] |
| 10. | zur Hausen A, Brink AA, Craanen ME, Middeldorp JM, Meijer CJ, van den Brule AJ. Unique transcription pattern of Epstein-Barr virus (EBV) in EBV-carrying gastric adenocarcinomas: expression of the transforming BARF1 gene. Cancer Res. 2000;60:2745-2748. [PubMed] |
| 11. | Korsmeyer SJ. Bcl-2 initiates a new category of oncogenes: regulators of cell death. Blood. 1992;80:879-886. [PubMed] |
| 12. | Ke H, Pei J, Ni Z, Xia H, Qi H, Woods T, Kelekar A, Tao W. Putative tumor suppressor Lats2 induces apoptosis through downregulation of Bcl-2 and Bcl-x(L). Exp Cell Res. 2004;298:329-338. [PubMed] [DOI] |
| 13. | Rohn JL, Noteborn MH. The viral death effector Apoptin reveals tumor-specific processes. Apoptosis. 2004;9:315-322. [PubMed] [DOI] |
| 14. | Zhao L, Guo QL, You QD, Wu ZQ, Gu HY. Gambogic acid induces apoptosis and regulates expressions of Bax and Bcl-2 protein in human gastric carcinoma MGC-803 cells. Biol Pharm Bull. 2004;27:998-1003. [PubMed] [DOI] |
| 15. | Kume T, Oshima K, Shinohara T, Takeo H, Yamashita Y, Shirakusa T, Kikuchi M. Low rate of apoptosis and overexpression of bcl-2 in Epstein-Barr virus-associated gastric carcinoma. Histopathology. 1999;34:502-509. [PubMed] [DOI] |
| 16. | Ohfuji S, Osaki M, Tsujitani S, Ikeguchi M, Sairenji T, Ito H. Low frequency of apoptosis in Epstein-Barr virus-associated gastric carcinoma with lymphoid stroma. Int J Cancer. 1996;68:710-715. [PubMed] [DOI] |
| 17. | Ishii H, Gobé G, Kawakubo Y, Sato Y, Ebihara Y. Interrelationship between Epstein-Barr virus infection in gastric carcinomas and the expression of apoptosis-associated proteins. Histopathology. 2001;38:111-119. [PubMed] [DOI] |
| 18. | Sheu LF, Chen A, Lee HS, Hsu HY, Yu DS. Cooperative interactions among p53, bcl-2 and Epstein-Barr virus latent membrane protein 1 in nasopharyngeal carcinoma cells. Pathol Int. 2004;54:475-485. [PubMed] [DOI] |
| 19. | Qi ZL, Zhao T, Zhou XH, Zhang JH, Han XQ, Zhu MG. Expressions of latent membrane protein 1, p53 and bcl-2 proteins and their significance in Hodgkin's lymphoma. Di Yi Jun Yi Da Xue Xue Bao. 2003;23:225-227. [PubMed] |
| 20. | Zhang X, Hu L, Fadeel B, Ernberg IT. Apoptosis modulation of Epstein-Barr virus-encoded latent membrane protein 1 in the epithelial cell line HeLa is stimulus-dependent. Virology. 2002;304:330-341. [PubMed] [DOI] |
| 21. | Sarac S, Akyol MU, Kanbur B, Poyraz A, Akyol G, Yilmaz T, Sungur A. Bcl-2 and LMP1 expression in nasopharyngeal carcinomas. Am J Otolaryngol. 2001;22:377-382. [PubMed] [DOI] |
| 22. | Chou SP, Tsai CH, Li LY, Liu MY, Chen JY. Characterization of monoclonal antibody to the Epstein-Barr virus BHRF1 protein, a homologue of Bcl-2. Hybrid Hybridomics. 2004;23:29-37. [PubMed] [DOI] |
| 23. | Huang Q, Petros AM, Virgin HW, Fesik SW, Olejniczak ET. Solution structure of the BHRF1 protein from Epstein-Barr virus, a homolog of human Bcl-2. J Mol Biol. 2003;332:1123-1130. [PubMed] [DOI] |
| 24. | Nicholls J, Kremmer E, Meseda CA, Mackett M, Hahn P, Gulley ML, Brink A, Swinnen LJ, Greenspan J, De Souza Y. Comparative analysis of the expression of the Epstein-Barr virus (EBV) anti-apoptotic gene BHRF1 in nasopharyngeal carcinoma and EBV-related lymphoid diseases. J Med Virol. 2001;65:105-113. [PubMed] [DOI] |
| 25. | Horner D, Lewis M, Farrell PJ. Novel hypotheses for the roles of EBNA-1 and BHRF1 in EBV-related cancers. Intervirology. 1995;38:195-205. [PubMed] [DOI] |
| 26. | Gulley ML, Pulitzer DR, Eagan PA, Schneider BG. Epstein-Barr virus infection is an early event in gastric carcinogenesis and is independent of bcl-2 expression and p53 accumulation. Hum Pathol. 1996;27:20-27. [PubMed] [DOI] |
| 27. | Tao Q, Srivastava G, Loke SL, Ho FC. Lack of correlation between expression of Epstein-Barr virus (EBV) latent membrane protein and bcl-2 oncoprotein in vivo. J Clin Pathol. 1994;47:589-591. [PubMed] [DOI] |
| 28. | Wei MX, de Turenne-Tessier M, Decaussin G, Benet G, Ooka T. Establishment of a monkey kidney epithelial cell line with the BARF1 open reading frame from Epstein-Barr virus. Oncogene. 1997;14:3073-3081. [PubMed] [DOI] |