修回日期: 2003-10-02
接受日期: 2003-11-19
在线出版日期: 2004-08-15
目的: 研究瘦素和雌激素在成年男性非酒精性脂肪肝病(NAFLD)发病中的作用.
方法: 采用放射免疫法(RIA)测定体检人群中肥胖合并NAFLD患者(n = 29)、单纯性肥胖者(n = 29)和正常对照者(n = 29)血清胰岛素、雌激素和瘦素水平, 同时采用稳态模型法计算胰岛素抵抗指数(HOMA-IRI).
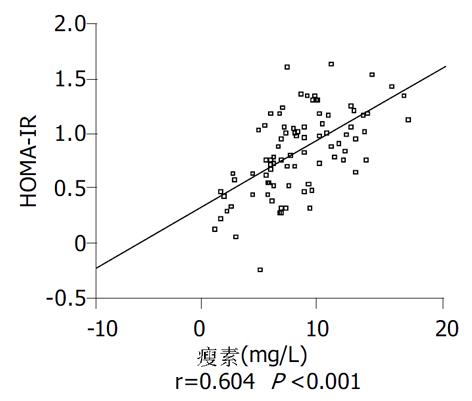
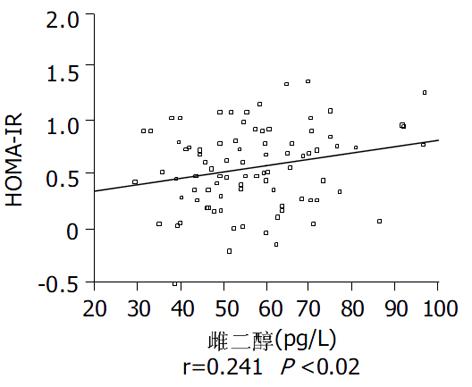
结果: 空腹胰岛素水平和HOMA-IRI在对照组、肥胖组和NAFLD三组, 依次升高, 存在显著性差异(P<0.05); NAFLD组瘦素水平为9.55±3.56 mg/L与肥胖组的8.07±2.92 mg/L相比较, 有升高的倾向(P = 0.124), 这两组均显著高于对照组5.17±3.29 mg/L (P<0.05); NAFLD组雌二醇为61.84±16.21 pg/L, 显著高于对照组的51.45±12.31 pg/L(P<0.05). HOMA-IRI与瘦素显著正相关(r = 0.604, P<0.001); 与雌二醇也呈显著正相关(r = 0.241, P = 0.02).
结论: NAFLD组的HOMA-IRI和空腹胰岛素水平高于肥胖组, 肥胖组高于对照组, 说明胰岛素抵抗是NAFLD患者基本特征; NAFLD患者瘦素和雌二醇水平升高, 提示瘦素和雌激素可能参与了其胰岛素抵抗的发生发展.
引文著录: 张桂英, 聂磊. 瘦素和雌激素在成年男性非酒精性脂肪肝病发病中的作用. 世界华人消化杂志 2004; 12(8): 1897-1899
Revised: October 2, 2003
Accepted: November 19, 2003
Published online: August 15, 2004
AIM: To investigate the role of estrogen and leptin in the pathogenesis of nonalcoholic fatty liver disease (NAFLD) in male adults.
METHODS: Serum estradiol and leptin were measured by radioimmunoassay (RIA) in the following 3 groups of patients: obesity with NAFLD (n = 29), obesity (n = 29), and healthy control (n = 29).
RESULTS: Fasting insulin concentration and insulin resistance index calculated by homeostasis model assessment (HOMA-IRI) were the highest in NAFLD group while the lowest in control group (P < 0.05). The levels of leptin in NAFLD group and obesity group were 9.55±3.56 mg/L, 8.07±2.92 mg/L, respectively, which were significantly higher than 5.17±3.29 mg/L in control group (P < 0.05). Estradiol concentration in NAFLD group was 61.84±16.21 pg/L, higher than 51.45±12.31 pg/L in control group (P < 0.05). HOMA-IRI was positively correlated with leptin (r = 0.604, P < 0.001) and estradiol (r = 0.241, P = 0.02).
CONCLUSION: NAFLD is characterized with insulin resistance. Serum estradiol and leptin are higher in NAFLD group than those in the other two groups, and they may be involved in the pathogenesis of insulin resistance.
- Citation: Zhang GY, Nie L. Leptin and estrogen in pathogenesis of nonalcoholic fatty liver disease in male adults. Shijie Huaren Xiaohua Zazhi 2004; 12(8): 1897-1899
- URL: https://www.wjgnet.com/1009-3079/full/v12/i8/1897.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v12.i8.1897
非酒精性脂肪肝病(nonalcoholic fatty liver disease, NAFLD)指病变主体在肝小叶、以肝细胞脂肪变性和脂肪沉积为病理特征但无过量饮酒史的临床综合征, 包括从单纯脂肪肝到脂肪肝炎、肝纤维化, 最后至肝硬化三种类型[1-2]. 肥胖是NAFLD发生的重要危险因素, 因肥胖常与胰岛素抵抗相关[3-4], 而胰岛素抵抗是NAFLD发生发展的基础[5-8]. 脂肪组织具有复杂的内分泌功能[9], 肥胖基因(ob gene)的表达产物瘦素(leptin)就是由脂肪细胞分泌的[10], 同时脂肪组织也是男性雌激素生成的主要场所, 来源于肾上腺皮质的雄烯二酮经脂肪组织芳香化酶作用生成雌酮, 再还原为具有生物活性的雌二醇[11]. 但有关瘦素和雌激素对肝脏脂肪变性的影响尚无定论. 我们测定了成年男性单纯性肥胖合并NAFLD (NAFLD组), 单纯性肥胖(肥胖组)以及正常对照(对照组)的血清胰岛素、瘦素和雌激素水平, 旨在探讨上述激素在成年男性NAFLD发病中的作用.
在湘雅医院门诊部体检人群中, 抽取符合标准的男性体检者, 每组各29例, 年龄25-55岁. NAFLD组平均年龄41.1±7.4岁, 体重指数(BMI)平均27.45±1.21 kg/m2. 肥胖组平均年龄40.3±7.6岁, BMI平均26.96±1.26 kg/m2. 对照组平均年龄39.7±6.3岁, BMI平均22.80±1.88 kg/m2. 三组年龄相当(P = 0.112); 肥胖组与NAFLD组相比BMI无显著差异(t = 1.288, P = 0.205). 以上所有受试者均无肝病史, 无服用可引起脂肪肝药物史, 饮酒量每周小于40 g酒精, 无糖尿病及无其他疾病病史, 肾功能正常. 对照组肝功能正常, 肥胖组与NAFLD组除谷丙转氨酶(ALT)外, 其余肝功能指标均正常, 肥胖组ALT均值37.69±20.24 IU/L, NAFLD组ALT 均值52.72±19.15 IU/L.
所有受试者均脱外衣, 脱鞋, 同一体重计, 同一标尺, 测量身高、体重、臀围和腰围. BMI = 体重(kg)/身高2(m), 腰臀比值(WHR)=腰围/臀围. 除常规健康体检之外, 对受试者清晨空腹抽静脉血检测肝肾功能、胰岛素、雌二醇和瘦素. 血清胰岛素、瘦素和雌二醇均以放射免疫法测定(试剂盒购自中国原子能科学研究院同位素研究所). 采用稳态模型法(homeostasis model assessment, HOMA)计算胰岛素抵抗指数(insulin resistance index, IRI). 公式为: 空腹血糖(FBS)×空腹胰岛素(INS)/22.5, 计算值取自然对数变换后, 成为正态分布[12].
统计学处理 采用SPSS10.0软件包分析. 以mean±SD表示. 数据进行正态性或方差齐性检验、单因素方差分析(ANOVA)、多个样本均数间两两比较(Newman-Keuls法)和相关分析. 以P<0.05 为差异有显著性.
NAFLD组腰臀比值(WHR)显著高于肥胖组, 肥胖组显著高于对照组(P<0.05); NAFLD组血糖和ALT水平显著高于对照组(P<0.05)(表1).
NAFLD组胰岛素水平和HOMA-IRI显著高于肥胖组, 肥胖组显著高于对照组(P均<0.05); NAFLD组雌二醇水平显著高于对照组(P<0.05); NAFLD组和肥胖组的瘦素水平显著高于对照组(P均<0.05), 与肥胖组相比较, NAFLD组有升高的倾向(P = 0.124)(表2).
胰岛素抵抗是NAFLD发生发展的基础[5-8]. 我们发现NAFLD组胰岛素水平和HOMA-IRI显著高于肥胖组和对照组, 且NAFLD患者胰岛素抵抗程度较相同BMI的肥胖无脂肪肝者更严重, 这说明胰岛素抵抗是NAFLD的基本特征. NAFLD的发生与脂肪分布类型有关[13-14]. 徐芸 et al[13]通过CT来计算腹内脂肪面积与肝脏脂肪浸润之间的关系, 结果发现中央型肥胖(腹内型肥胖)是NAFLD最重要的危险因素之一; Nakao et al[14]通过B超测腹壁厚度, 发现腹内型肥胖是NAFLD发生的惟一危险因素; Ruderman et al[15]在BMI正常的患者中也发现了相同的结果. 我们也发现NAFLD组的腰臀比值(WHR)显著大于BMI相同的肥胖组, 这说明两组间脂肪分布类型存在差异, 中央型肥胖才是NAFLD的危险因素.
研究表明, 瘦素可能具有从外周直接抗肝脂肪变性的作用[16]. 瘦素可以激活AMP活化蛋白激酶(AMP-activated protein kinase)减少脂肪沉积[17], 还可特异性抑制肝脏的硬脂酰基-辅酶A去饱和酶 (stearoyl-CoA desaturase-1), 使肝脏VLDL 生成和脂肪沉积减少[18], 已发现瘦素替代治疗对于脂质营养不良的患者可以减轻肝脏脂肪沉积[19]. 但是Chitturi et al[20]发现NAFLD患者有高瘦素血症, 且瘦素水平与肝脏的脂肪变性程度直接正相关. 另最近Chalasani et al[21]报道, 瘦素水平与脂肪肝的发生并无直接关系. 因此, 瘦素对肝脏脂肪变性的影响尚无定论. 本研究结果显示NAFLD组瘦素水平与肥胖组相比有升高的倾向, 但无统计学意义.
瘦素对胰岛素抵抗的影响较为复杂. 瘦素可直接作用于周围器官和组织(骨骼肌、肝脏、胰岛和脂肪组织)对葡萄糖代谢起抑制作用, 促进胰岛素抵抗[22], 瘦素又可通过中枢神经系统机制来调节葡萄糖代谢, 促进胰岛素敏感性[23], 整体水平观察瘦素对胰岛素抵抗影响时, 应综合考虑以上两方面作用. 肥胖者瘦素水平升高, 可能超过了血脑屏障转运的阈值[24], 此时瘦素介导的中枢神经系统效应无相应增加, 高水平的瘦素对周围器官组织的作用变得明显, 因此瘦素的作用以促进胰岛素抵抗为主[25]. 我们发现HOMA-IRI与瘦素水平显著正相关, 提示瘦素有可能促进了NAFLD患者的胰岛素抵抗, 引起肝脏脂肪变性.
肥胖者脂肪细胞增大, 数目增加, 雌激素产生增加[11], 本研究也发现, 在NAFLD组和肥胖组中雌二醇浓度升高. 有关雌激素与脂肪肝的关系较为复杂, 一方面, 雌激素水平过低也会促进脂肪肝的发生. Jones et al[26] 发现, 芳香化酶基因敲除鼠(ArKO 小鼠)因不能合成内源性雌激素, 而出现其肝脏中脂质沉积; 且还发现雌激素在维持肝脏脂质β-氧化酶系基因组成性表达和维持肝脏脂质稳态中也起关键作用[27]. 我们在流行病学调查中也发现更年期女性雌激素分泌水平下降, 而NAFLD患病率升高, 已有报道使用小剂量激素替代治疗的妇女其NAFLD患病率要低于未使用者[28]. 另一方面, 升高的雌激素水平可因抑制肝脏脂肪酸氧化、促进脂肪酸合成[29]和损伤线粒体[30]导致脂肪肝. 本研究中, 我们发现成年男性NAFLD患者其雌二醇水平显著高于对照组, 且与HOMA-IRI显著正相关, 提示在成年男性中, 升高的雌激素还可能通过促进胰岛素抵抗导致脂肪肝.
编辑: N/A
| 1. | Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221-1231. [PubMed] [DOI] |
| 3. | Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest. 2000;106:473-481. [PubMed] [DOI] |
| 5. | Marchesini G, Brizi M, Bianchi G, Tomassetti S, Bugianesi E, Lenzi M, McCullough AJ, Natale S, Forlani G, Melchionda N. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. 2001;50:1844-1850. [PubMed] [DOI] |
| 7. | Brunt EM. Nonalcoholic steatohepatitis: definition and pathology. Semin Liver Dis. 2001;21:3-16. [PubMed] [DOI] |
| 8. | Marchesini G, Brizi M, Morselli-Labate AM, Bianchi G, Bugianesi E, McCullough AJ, Forlani G, Melchionda N. Association of nonalcoholic fatty liver disease with insulin resistance. Am J Med. 1999;107:450-455. [PubMed] [DOI] |
| 10. | Ahima RS, Flier JS. Leptin. Annu Rev Physiol. 2000;62:413-437. [PubMed] [DOI] |
| 11. | Nelson LR, Bulun SE. Estrogen production and action. J Am Acad Dermatol. 2001;45:S116-S124. [PubMed] [DOI] |
| 12. | 任 颖, 陆 广华, 厉 锦华, 周 桓, 范 吴强, 黄 定九. HOMA法和血糖钳夹法胰岛素抵抗指数的关系. 上海第二医科大学学报. 2002;22:325-326. |
| 14. | Nakao K, Nakata K, Ohtsubo N, Maeda M, Moriuchi T, Ichikawa T, Hamasaki K, Kato Y, Eguchi K, Yukawa K. Association between nonalcoholic fatty liver, markers of obesity, and serum leptin level in young adults. Am J Gastroenterol. 2002;97:1796-1801. [PubMed] [DOI] |
| 15. | Ruderman N, Chisholm D, Pi-Sunyer X, Schneider S. The metabolically obese, normal-weight individual revisited. Diabetes. 1998;47:699-713. [PubMed] [DOI] |
| 16. | Unger RH. Lipotoxic diseases. Annu Rev Med. 2002;53:319-336. [PubMed] [DOI] |
| 17. | Minokoshi Y, Kim YB, Peroni OD, Fryer LG, Müller C, Carling D, Kahn BB. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002;415:339-343. [PubMed] [DOI] |
| 18. | Cohen P, Miyazaki M, Socci ND, Hagge-Greenberg A, Liedtke W, Soukas AA, Sharma R, Hudgins LC, Ntambi JM, Friedman JM. Role for stearoyl-CoA desaturase-1 in leptin-mediated weight loss. Science. 2002;297:240-243. [PubMed] [DOI] |
| 19. | Oral EA, Simha V, Ruiz E, Andewelt A, Premkumar A, Snell P, Wagner AJ, DePaoli AM, Reitman ML, Taylor SI. Leptin-replacement therapy for lipodystrophy. N Engl J Med. 2002;346:570-578. [PubMed] [DOI] |
| 20. | Chitturi S, Farrell G, Frost L, Kriketos A, Lin R, Fung C, Liddle C, Samarasinghe D, George J. Serum leptin in NASH correlates with hepatic steatosis but not fibrosis: a manifestation of lipotoxicity? Hepatology. 2002;36:403-409. [PubMed] [DOI] |
| 21. | Chalasani N, Crabb DW, Cummings OW, Kwo PY, Asghar A, Pandya PK, Considine RV. Does leptin play a role in the pathogenesis of human nonalcoholic steatohepatitis? Am J Gastroenterol. 2003;98:2771-2776. [PubMed] [DOI] |
| 22. | Sweeney G, Keen J, Somwar R, Konrad D, Garg R, Klip A. High leptin levels acutely inhibit insulin-stimulated glucose uptake without affecting glucose transporter 4 translocation in l6 rat skeletal muscle cells. Endocrinology. 2001;142:4806-4812. [PubMed] [DOI] |
| 23. | Minokoshi Y, Haque MS, Shimazu T. Microinjection of leptin into the ventromedial hypothalamus increases glucose uptake in peripheral tissues in rats. Diabetes. 1999;48:287-291. [PubMed] [DOI] |
| 24. | Wang ZW, Zhou YT, Kakuma T, Lee Y, Higa M, Kalra SP, Dube MG, Kalra PS, Unger RH. Comparing the hypothalamic and extrahypothalamic actions of endogenous hyperleptinemia. Proc Natl Acad Sci U S A. 1999;96:10373-10378. [PubMed] [DOI] |
| 25. | Ceddia RB, Koistinen HA, Zierath JR, Sweeney G. Analysis of paradoxical observations on the association between leptin and insulin resistance. FASEB J. 2002;16:1163-1176. [PubMed] [DOI] |
| 26. | Jones ME, Thorburn AW, Britt KL, Hewitt KN, Wreford NG, Proietto J, Oz OK, Leury BJ, Robertson KM, Yao S. Aromatase-deficient (ArKO) mice have a phenotype of increased adiposity. Proc Natl Acad Sci U S A. 2000;97:12735-12740. [PubMed] [DOI] |
| 27. | Nemoto Y, Toda K, Ono M, Fujikawa-Adachi K, Saibara T, Onishi S, Enzan H, Okada T, Shizuta Y. Altered expression of fatty acid-metabolizing enzymes in aromatase-deficient mice. J Clin Invest. 2000;105:1819-1825. [PubMed] [DOI] |
| 28. | Harrison SA, Diehl AM. Fat and the liver--a molecular overview. Semin Gastrointest Dis. 2002;13:3-16. [PubMed] |
| 29. | O'Sullivan AJ, Martin A, Brown MA. Efficient fat storage in premenopausal women and in early pregnancy: a role for estrogen. J Clin Endocrinol Metab. 2001;86:4951-4956. [PubMed] [DOI] |
| 30. | Grimbert S, Fisch C, Deschamps D, Berson A, Fromenty B, Feldmann G, Pessayre D. Effects of female sex hormones on mitochondria: possible role in acute fatty liver of pregnancy. Am J Physiol. 1995;268:G107-G115. [PubMed] |