修回日期: 2004-03-12
接受日期: 2004-04-13
在线出版日期: 2004-08-15
目的: 探讨大鼠肝部分切除术后肝窦血管内皮细胞 (LSEC)对再生肝细胞增生的影响.
方法: 建立Wistar 大鼠70%的肝切除模型和肝窦血管内皮细胞、肝细胞分离与培养方法, 在术后不同的时相点分离残肝的肝细胞和肝窦血管内皮细胞, 进行培养. 实验分组: 肝部分切除组(PO)和假手术组(SO); 肝细胞培养分两组: A组(肝细胞)、B组(肝细胞+LSEC上清液), 利用放射免疫法测定肝细胞DNA合成率、免疫组化方法测定肝细胞PCNA表达指数的变化.
结果: 在术后的6、24 h, B组的肝细胞的 PCNA表达指数较A组显著升高(5.9±0.1 vs 8.9±0.1 P<0.05; 38.6±2.6 vs 58.0±3.9 P<0.01). 而且B组肝细胞的DNA合成量较A组显著升高(226±18 vs 8.9±0.1 P<0.05; 38.6±2.6 vs 58.0±3.9 P<0.01).
结论: 体外实验证实肝部分切除术后肝窦内皮细胞上清可以促进肝细胞的增生, 促进肝细胞的DNA合成, 增强再生肝细胞增生活性.
引文著录: 董晓灵, 陈平, 朱瑾, 熊燕. 大鼠肝部分切除术后肝窦内皮细胞对肝细胞增生的影响. 世界华人消化杂志 2004; 12(8): 1861-1864
Revised: March 12, 2004
Accepted: April 13, 2004
Published online: August 15, 2004
AIM: To explore the effects of liver sinusoidal endothelial cells on the proliferation of the regenerative hepatocytes after partial hepatectomy in normal rats.
METHODS: A method to separate and culture hepatocytes and liver sinusoidal endothelial cells (LSEC) was established and the hepacytes and sinusoidal endothelial cells of regenerative liver were separated and cultured at different time points after partial hepaectomy in normal rats. The experiment was divided into two groups: operation group (OP) and sham operation group (SO). The cultivation of hepatocytes was divided into two groups: group A (hepatocytes) and group B (hepatocytes + supernatant of LSEC). The expression index of PCNA in hepatocytes was assayed by immunohistochemistry, and the level of synthesis of DNA in regenerative hepatocytes was assayed by radio-immunity.
RESULTS: The expression index of PCNA in hepatocytes cultured in group B increased more significantly than that in group A after partial hepatectomy in 6h and 24 h (5.9±0.1 vs 8.9±0.1 P < 0.05; 38.6±2.6 vs 58.0±3.9 P < 0.01),and so did the level of synthesis of DNA in regenerating hepatocytes cultured in group B than that in group A after partial hepatectomy in 6 h and 24 h (226±18 vs 8.9±0.1 P < 0.05; 38.6±2.6 vs 58.0±3.9 P < 0.01).
CONCLUSION: The proliferating ability and the synthesis of DNA in hepatocytes after partial hepatectomy are enhanced by the supernatant from LSEC in vitro.
- Citation: Dong XL, Chen P, Zhu J, Xiong Y. Effects of liver sinusoidal endothelial cells on proliferation of hepatocytes after partial hepatectomy in rats. Shijie Huaren Xiaohua Zazhi 2004; 12(8): 1861-1864
- URL: https://www.wjgnet.com/1009-3079/full/v12/i8/1861.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v12.i8.1861
肝再生是肝叶部分切除术后最基本的病理过程, 非实质性细胞发挥了重要作用, 肝窦内皮细胞(LSEC)在肝再生中的作用是近年来越来越受到人们重视, 增生细胞核抗原(PCNA)表达可以反应出肝细胞内DNA的合成及肝细胞增生的活性. 我们通过复制大鼠肝部分切除术模型, 建立稳定的肝细胞、肝窦内皮细胞的分离与培养方法, 通过细胞培养, 试图观察肝窦内皮细胞对再生肝肝细胞增生的影响, 进一步探讨LSEC肝再生过程中的作用.
♂Wistar 大鼠, 质量250-300 g, 购自本校实验动物中心. Percoll 购自Pharmaica Fine Chemica; Ⅳ型胶原酶、Dnase、GBSS均为Sigma公司产品; DMEM, D-Hanks, RPMI 1640均为Gibco公司产品; PCNA检测试剂盒为博士德产品; 小牛血清购自上海生物制品有限公司; 3H-胸腺嘧啶核苷(3H-TDR)购自中科院原子能研究所. 大鼠肝部分切除术动物模型的建立, 采用Higgins and Aderson创建的经典大鼠肝部分切除术模型(Arch Pathol 1931; 12: 186): 即分别于肝左叶、中叶根部结扎切除, 本实验采用80只Wistar♂大鼠, 随机分到肝部分切除手术组(PH)和假手术组(SO)的5个时相点, 即0、6、24、48、72 h, 每个时相点8只, 保证每组每个时相点成活6只以上, 另外取10只用来做肝切除率测定, 得出平均切除率为69%±1.5%.
我们在采用BREAT法(Lab Invest 1994; 70: 944)的基础上稍作了改进, 简言之, 打开大鼠腹腔, 腹腔静脉肝素钠注射, 门静脉插管, 输液泵匀速前灌液原位灌注, 待肝脏血液冲洗干净后, 用0.5 g/L的胶原酶以8-10 mL/min的速度循环灌注肝脏10 min, 抖动肝脏分离肝细胞和非实质性细胞, 200目滤网过滤, Beckman低温离心机500 r/min离心8 min, 沉淀肝细胞(用作细胞培养), 上清以×100g离心10 min, 进一步除去肝细胞, 得到肝非实质性细胞悬液, 以×400 g离心10 min, 得到非实质性细胞, 非实质性细胞用PBS 洗涤×400 g 离心10 min, PBS重悬, 进行50% Percoll, 25% Percoll密度梯度离心, 调整细胞浓度为1×107/L, 100 mL/L小牛血清RPMI 640培养, 肝细胞PBS洗涤500 r/min ×3次, 调整细胞密度为1×107/L, 100 mL/L小牛血清RPMI1640培养, 台盼蓝拒染实验检查细胞活性. 大鼠内皮细胞单抗免疫荧光染色, 倒置荧光显微镜下测定LSEC纯度. 将重悬的肝细胞调至浓度为1×109/L, 置于6孔板(内置多聚赖氨酸处理的细胞爬片), 和96孔板100 mL/L小牛血清RPMI1640培养8 h后, 分A、B两组, 6孔板上一排3孔为A组, 下一排3孔为B组, 96孔板选10复孔, 1-5孔为A组, 6-10孔为B组; A组: 肝细胞; B组: 肝细胞+SEC培养的上清液. SEC培养: 将重悬的细胞调整细胞浓度为1×1010/L, 100 mL/L小牛血清RM1640培养瓶中培养, 肝细胞培养8 h后, 换培养液, 取LSEC的上清液1 mL 加入到6孔板B组的每孔中, 往96孔板的B组每孔加入LSEC上清40 μL, 6孔板继续培养16 h, 收集肝细胞爬片, 40 g/L的多聚甲醛固定备用. 96孔板继续培养8 h, 每孔加入37 kbq 3H-TDR, 培养8 h后行DNA合成率测定.
1.2.1 肝细胞PCNA表达指数的测定: 采用ABC法, 按试剂盒方法步骤进行操作, 加入临时配制的DAB显色液, 显微镜下观察显色, 苏木素复染后, 树胶封片.
实验结果判定: PCNA阳性染色呈棕黄色颗粒, 主要分布在肝细胞核内, 在显微镜下随机观测10个视野/张爬片, 80-100个细胞/视野, 按下列公式计算PCNA的指数: PCNA阳性肝细胞数÷观察的肝细胞总数×100%.
1.2.2 大鼠肝细胞DNA合成率的测定: 用多头细胞收集仪收集96孔板每孔细胞于玻璃纤维膜上, 先后用生理盐水, 50 g/L三氯醋酸洗涤去除游离3H-TDR, 无水乙醇脱水, 烤干冷却后放入5 μL闪烁液中, 于LKB-1217型闪烁仪上测CPM值. 以CPM/105cells表示DNA的合成率.
统计学处理 资料表达采用mean±SD, 用Microsoft excel、Spss10.0软件在微机上进行单因素方差分析, t检验.
LSEC细胞得率都在1.0×1010/L以上, Percoll密度梯度离心法分离的LSEC细胞活性较细胞淘洗法明显增高(0.94±0.02 vs 0.87±0.03, P<0.01), 在统计学上有非常显著差异, LSEC细胞纯度也教较细胞淘洗法高(0.91±0.02 vs 0.86±0.03, P<0.05), 在统计学上有显著差异.
在假手术组中A组与B组的肝细胞PCNA的表达指数在统计学上没有显著差异(P>0.05), 在肝部分切除(PH)组6、24、48、72 h各个时相点B组的 PCNA表达指数均较A组增高(表1), 其中在6 h 点P<0.05, 24 h点P<0.01在统计学上有显著意义.
在术后6、24、48、72 h各个时相点B组肝细胞的DNA合成率均较A组升高, 在6 h两组间比较(242±17 vs 85±2.4 μBq/cell P<0.05), 在24 h两组间比较(310±26 vs 139±17, μBq/cell P<0.01), 48、72 h点两组间比较(P>0.05).
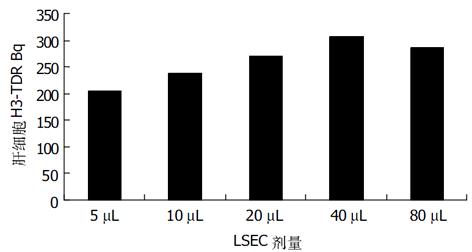
在术后24 h 3H-TDR Bq与加入的LSEC量呈线形关系, 在96孔板每孔加入40 μL时达到高峰(图1).
肝再生的研究对肝功能衰竭的预防、肝功能的恢复及指导外科肝切除术具有非常重要的意义. 肝再生过程和肝再生机制非常复杂, 如再生如何启动又如何终止?目前仍不清楚. 肝再生虽然以研究肝细胞的增生为主, 但肝非实质细胞发挥的作用越来越受到人们的关注. 肝窦内皮细胞, 在所有非实质性细胞中所占的比例最大, 约50%, 与肝脏微循环、内环境的稳定、肝脏血脂代谢、内分泌代谢、肝再生、肝纤维化、肝癌的发生与转移, 肝移植等均有密切关系[1-7]. 在肝再生中研究表明: 肝部分切除术后最为明显、最先出现肝脏血流的改变, 单位重量的肝组织内血流急剧升高数倍或十多倍, 认为这种变化可能是肝再生启动的始发因素[8-12].
肝窦内皮细胞(LSEC)是肝细胞增生的"感受器"由于位置特殊, 即肝窦, 能最先感受血流的急剧变化而导致剪切力的改变, 从而激活分泌肝细胞生长因子(HGF)、肿瘤坏死因子-α(TNF-α)、转化生长因子-β(TGF-β), 血管内皮生长因子(VEGF)、内皮素、白介素-6(IL-6)、一氧化氮合酶等[13-18]. 正是由于这些细胞生长因子和细胞因子在肝再生中发挥了重要的作用, 因此肝窦内皮细胞在肝再生中的作用越来越受到人们的关注. 目前HGF作为一种肝细胞丝裂原物质能促进肝细胞的分裂与增生, 在肝再生的研究中研究最多, 在体内、体外的实验均证实这种作用[19-21]. LSEC作为潜在的肝细胞增生的调节器, 更为重要的是在肝切除术后早期LSEC中TNF-α表达的明显增加, 而TNF-α、IL-6是肝细胞再生启动的细胞因子, TNF通过与TNFR-1结合激活NFκβ, IL-6激活STAT3, 活化肝细胞, 使之突破G1/S的制约点, 使其对生长因子具有充分的反应性[22-25]. Cressman et al研究表明肝部分切除术后IL-6主要来源于肝脏非实质性细胞和肠道, IL-6在肝细胞增生启动方面发挥了重要作用, IL-6及TNF-α在正常大鼠肝部分切除术后血中浓度均升高, 并且单纯注射IL-6能够纠正TNFR-1基因敲除的大鼠肝再生受阻及STAT3活化障碍, Cressman et al研究表明在IL-6基因敲除的小鼠, 肝再生及STAT3活化明显损害, 给予IL-6能纠正该现象. 研究表明IL-6+受体结合→活化NF-κβ→活化STAT3→促进即刻早期基因的表达→启动和促进肝再生[22,26-29].
本实验中, 我们采用同一性别即♂Wistar 大鼠是为了避免由于不同性别的性激素的差异对肝细胞增生的影响, 通过免疫组化法测定肝切除后肝细胞的PCNA表达指数和放射免疫法测定3H-TDR掺入率来测定肝细胞DNA合成, 结果都显示在术后6 h, LSEC上清能促进肝细胞DNA合成, 在24 h达到高峰, 并且发现这种促进作用与加入的LSEC的上清的量呈线形关系, 而且这种促进作用与手术后时间有关, 在术后早期即6、24 h表现明显, 在48 h后这种促进作用减退, 同时LSEC上清对肝细胞的DNA合成促进作用也与肝切除术有关, 在假手术组LSEC上清对肝细胞的DNA合成没有显著地促进作用, 初步表明大鼠肝部分切除术后LSEC通过旁分泌作用明显促进肝细胞DNA的合成, 增加再生肝细胞的增生活性.
我们分析可能是肝部分切除术, 导致肝内微环境的急剧变化, 通过某种方式激活LSEC, 通过分泌产生一种循环物质, 这种物质能够在早期促进肝细胞的DNA合成, 促进肝细胞分裂. 这种物质可能与LSEC产生的HGF、IL-6、TNF-α等有关, 至于LSEC如何启动肝细胞及是否通过分泌HGF及其他的细胞因子来促进肝细胞DNA的合成, 有待于进一步研究.
编辑: N/A
| 1. | Xu B, Broome U, Uzunel M, Nava S, Ge X, Kumagai-Braesch M, Hultenby K, Christensson B, Ericzon BG, Holgersson J. Capillarization of hepatic sinusoid by liver endothelial cell-reactive autoantibodies in patients with cirrhosis and chronic hepatitis. Am J Pathol. 2003;163:1275-1289. [PubMed] [DOI] |
| 2. | LeCouter J, Moritz DR, Li B, Phillips GL, Liang XH, Gerber HP, Hillan KJ, Ferrara N. Angiogenesis-independent endothelial protection of liver: role of VEGFR-1. Science. 2003;299:890-893. [PubMed] [DOI] |
| 3. | Nedredal GI, Elvevold KH, Ytrebø LM, Olsen R, Revhaug A, Smedsrød B. Liver sinusoidal endothelial cells represents an important blood clearance system in pigs. Comp Hepatol. 2003;2:1. [PubMed] [DOI] |
| 4. | Limmer A, Knolle PA. Liver sinusoidal endothelial cells: a new type of organ-resident antigen-presenting cell. Arch Immunol Ther Exp (Warsz). 2001;49 Suppl 1:S7-11. [PubMed] |
| 5. | Limmer A, Ohl J, Kurts C, Ljunggren HG, Reiss Y, Groettrup M, Momburg F, Arnold B, Knolle PA. Efficient presentation of exogenous antigen by liver endothelial cells to CD8+ T cells results in antigen-specific T-cell tolerance. Nat Med. 2000;6:1348-1354. [PubMed] [DOI] |
| 6. | Mendoza L, Vidal-Vanaclocha F. Inflammatory response of tumor-activated hepatic sinusoidal endothelium as a target for the screening of metastasis chemopreventive drugs. Methods Mol Med. 2003;85:107-115. [PubMed] |
| 7. | Mendoza L, Carrascal T, De Luca M, Fuentes AM, Salado C, Blanco J, Vidal-Vanaclocha F. Hydrogen peroxide mediates vascular cell adhesion molecule-1 expression from interleukin-18-activated hepatic sinusoidal endothelium: implications for circulating cancer cell arrest in the murine liver. Hepatology. 2001;34:298-310. [PubMed] [DOI] |
| 8. | Ito T, Matsuda Y, Oguchi Y, Ito T, Matsuda H. Evaluation of portal vascular resistance using an intraoperative Doppler ultrasound. Hepatogastroenterology. 2002;49:501-503. [PubMed] |
| 9. | Kaneko T, Sugimoto H, Inoue S, Tezel E, Ando H, Nakao A. Doppler ultrasonography for postoperative hepatofugal portal blood flow of the intrahepatic portal vein. Hepatogastroenterology. 2003;50:1825-1829. [PubMed] |
| 10. | Kahn D, van Hoorn-Hickman R, Terblanche J. Liver blood flow after partial hepatectomy in the pig. J Surg Res. 1984;37:290-294. [PubMed] [DOI] |
| 11. | Schoen JM, Wang HH, Minuk GY, Lautt WW. Shear stress-induced nitric oxide release triggers the liver regeneration cascade. Nitric Oxide. 2001;5:453-464. [PubMed] [DOI] |
| 12. | Sato Y, Tsukada K, Hatakeyama K. Role of shear stress and immune responses in liver regeneration after a partial hepatectomy. Surg Today. 1999;29:1-9. [PubMed] [DOI] |
| 13. | Malik R, Selden C, Hodgson H. The role of non-parenchymal cells in liver growth. Semin Cell Dev Biol. 2002;13:425-431. [PubMed] [DOI] |
| 14. | Hasmall SC, West DA, Olsen K, Roberts RA. Role of hepatic non-parenchymal cells in the response of rat hepatocytes to the peroxisome proliferator nafenopin in vitro. Carcinogenesis. 2000;21:2159-2165. [PubMed] [DOI] |
| 15. | Hasmall S, James N, Hedley K, Olsen K, Roberts R. Mouse hepatocyte response to peroxisome proliferators: dependency on hepatic nonparenchymal cells and peroxisome proliferator activated receptor alpha (PPARalpha). Arch Toxicol. 2001;75:357-361. [PubMed] [DOI] |
| 16. | Taniguchi E, Sakisaka S, Matsuo K, Tanikawa K, Sata M. Expression and role of vascular endothelial growth factor in liver regeneration after partial hepatectomy in rats. J Histochem Cytochem. 2001;49:121-130. [PubMed] [DOI] |
| 17. | Mochida S, Ishikawa K, Toshima K, Inao M, Ikeda H, Matsui A, Shibuya M, Fujiwara K. The mechanisms of hepatic sinusoidal endothelial cell regeneration: a possible communication system associated with vascular endothelial growth factor in liver cells. J Gastroenterol Hepatol. 1998;13 Suppl:S1-S5. [PubMed] [DOI] |
| 18. | Sato T, El-Assal ON, Ono T, Yamanoi A, Dhar DK, Nagasue N. Sinusoidal endothelial cell proliferation and expression of angiopoietin/Tie family in regenerating rat liver. J Hepatol. 2001;34:690-698. [PubMed] [DOI] |
| 19. | Michalopoulos G. Hepatocyte growth factor in liver growth and differentiation J Biol Chem 1995 In Strain AJ, Diehl AM, editors, Liver Growth and Repair. London: Chapman Hall 1998; 219-239. |
| 20. | Daveau M, Scotte M, François A, Coulouarn C, Ros G, Tallet Y, Hiron M, Hellot MF, Salier JP. Hepatocyte growth factor, transforming growth factor alpha, and their receptors as combined markers of prognosis in hepatocellular carcinoma. Mol Carcinog. 2003;36:130-141. [PubMed] [DOI] |
| 21. | Suzuki A, Iwama A, Miyashita H, Nakauchi H, Taniguchi H. Role for growth factors and extracellular matrix in controlling differentiation of prospectively isolated hepatic stem cells. Development. 2003;130:2513-2524. [PubMed] [DOI] |
| 22. | Koniaris LG, McKillop IH, Schwartz SI, Zimmers TA. Liver regeneration. J Am Coll Surg. 2003;197:634-659. [PubMed] [DOI] |
| 23. | Kirillova I, Chaisson M, Fausto N. Tumor necrosis factor induces DNA replication in hepatic cells through nuclear factor kappaB activation. Cell Growth Differ. 1999;10:819-828. [PubMed] |
| 24. | Iocca HA, Isom HC. Tumor necrosis factor-alpha acts as a complete mitogen for primary rat hepatocytes. Am J Pathol. 2003;163:465-476. [PubMed] [DOI] |
| 25. | Chen G, Cao P, Goeddel DV. TNF-induced recruitment and activation of the IKK complex require Cdc37 and Hsp90. Mol Cell. 2002;9:401-410. [PubMed] [DOI] |
| 26. | Li W, Liang X, Kellendonk C, Poli V, Taub R. STAT3 contributes to the mitogenic response of hepatocytes during liver regeneration. J Biol Chem. 2002;277:28411-28417. [PubMed] [DOI] |
| 27. | Cressman DE, Greenbaum LE, DeAngelis RA, Ciliberto G, Furth EE, Poli V, Taub R. Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science. 1996;274:1379-1383. [PubMed] [DOI] |
| 28. | Peters M, Blinn G, Jostock T, Schirmacher P, Meyer zum Büschenfelde KH, Galle PR, Rose-John S. Combined interleukin 6 and soluble interleukin 6 receptor accelerates murine liver regeneration. Gastroenterology. 2000;119:1663-1671. [PubMed] [DOI] |
| 29. | Debonera F, Aldeguer X, Shen X, Gelman AE, Gao F, Que X, Greenbaum LE, Furth EE, Taub R, Olthoff KM. Activation of interleukin-6/STAT3 and liver regeneration following transplantation. J Surg Res. 2001;96:289-295. [PubMed] [DOI] |