修回日期: 2004-02-21
接受日期: 2004-03-06
在线出版日期: 2004-08-15
目的: 研究热休克蛋白90抑制剂格尔德霉素(geldanamycin, GA)对肝癌细胞生长的影响并探讨相关机制.
方法: 用MTT法检测药物对肝癌HepG2细胞的生长抑制作用, 并用流式细胞术分析细胞周期与凋亡状况.Western印记杂交法检测细胞内蛋白激酶B/AKT磷酸化状态的变化.
结果: 400 μmoL/L浓度的GA处理细胞后, HepG2细胞胞内磷酸化AKT的水平明显下降(24 h为对照组66.03%, 48 h为对照组34.02%); GA作用后细胞呈现G2/M期阻滞并伴有凋亡率增加; 同时MTT法显示GA对HepG2细胞有生长抑制作用, 用药时间越长抑制率越高, 24 h和48 h的抑制率分别为4.09%和21.74%.
结论: 通过GA抑制Hsp90的功能能够抑制肝癌细胞的生长并促进其凋亡, 这一效应与信号传导系统活化状态的改变有关.
引文著录: 马昕, 林菊生, 杨志芳, 黎培员, 宋东坡. 格尔德霉素对肝癌细胞Akt磷酸化及细胞周期的影响. 世界华人消化杂志 2004; 12(8): 1793-1795
Revised: February 21, 2004
Accepted: March 6, 2004
Published online: August 15, 2004
AIM: To study the anti-proliferation effects of inhibitor of heat shock protein 90 (Hsp90), geldanamycin (GA), and its mechanism on human hepatoma HepG2 cells.
METHODS: MTT assay was used to detect the effect of growth inhibition of HepG2 cells.Cell cycle and apoptosis were analyzed by flow cytometry.Alteration of phosphorylated Akt was analyzed by Western blot assay.
RESULTS: GA significantly inhibited growth of HepG2 cells.After 24 or 48 h treatment with GA, the level of phosphorylated Akt was reduced significantly.It was about 66.0% (24 h) and 34.0% (48 h) compared to that of control cells.G2/M arrest was also prominent.The rate of apoptosis increased from 3.2% to 8.1% after 24 h treatment and from 4.0% to 11.42% after 48 h treatment, respectively.
CONCLUSION: The functional Hsp90 is important for the growth of hepatoma cells.It may be a promising way to cure liver cancer using the inhibitor of Hsp90.
- Citation: Ma X, Lin JS, Yang ZF, Li PY, Song DP. Effects of geldanamycin on PKB/Akt phosphorylation and cell cycle in human hepatoma HepG2 cells. Shijie Huaren Xiaohua Zazhi 2004; 12(8): 1793-1795
- URL: https://www.wjgnet.com/1009-3079/full/v12/i8/1793.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v12.i8.1793
热休克蛋白90(heat shock protein 90, Hsp90)是一种分子伴侣(chaperone)[1], 在保护蛋白质的正确空间结构、防止蛋白质意外降解、保证其功能方面有重要的意义[2-3].Hsp90在多种肿瘤组织中表达明显增高[3-4], 并对肿瘤的发生发展有重要意义, 目前已成为抗肿瘤研究的热门靶点[5].格尔德霉素(geldanamycin, GA)属于苯醌安莎霉素类抗生素, 是一种天然存在的Hsp90功能抑制剂[6], 能够阻止Hsp90发挥分子伴侣的功能, 是一种有望应用于临床的抗癌新药[7].我们的实验研究了GA对肝癌细胞生长的影响, 并对其机制进行了初步探讨, 以便为GA的临床应用提供理论依据.
GA为Alexis公司产品, 用DMSO(Sigma公司产品)溶解, 保存于-20 ℃.氮蓝四唑盐 (MTT)、碘化丙啶 (PI)为Biomol公司产品, 分别用磷酸盐缓冲液溶解成 5 g/L和 65 mg/L, 避光储存于4 ℃.兔抗磷酸化AKT单克隆抗体(Ser-473)购自CST公司, 二抗HRP标记羊抗兔IgG、化学发光(ECL)底物购自Santa Cruz公司.人肝癌细胞株HepG2购自武汉大学中国典型生物保藏中心, 高糖型DMEM培养基为GibcoBRL公司产品, 胎牛血清购自四季青公司.其余试剂均为市售分析纯.
1.2.1 细胞培养与MTT: 人肝癌细胞株HepG2用含10 mL/L胎牛血清的DMEM培养液培养于37 ℃, 50 mL/L CO2饱和湿度的培养箱中.收集对数生长期的细胞, 调整密度, 5 000个/孔接种于96孔培养板.继续培养24 h后加入GA储存液至终浓度为0.4 μmoL/L, 等量DMSO为空白对照, 每组设4个复孔.药物作用24 h或48 h后每孔加入预先配好的 MTT溶液10 μL, 再培养4 h, 弃去上清液, 用DMSO150 μL溶解沉淀, 微型振荡仪振荡10 min混匀, 用酶标以测定波长490 nm的吸光度 (A), 细胞抑制率 = (1- 试验孔A/ 对照孔A)×100%.实验重复3, 取平均值.
1.2.2 流式细胞术: 4 μmoL/LGA处理24或48 h后收集细胞, 用 -20 ℃预冷的80 mL/L乙醇固定过夜.固定后细胞用PBS洗涤2次, 用10 mg/L PI和1 g/L Rnase A, 在室温下避光进行DNA染色20 min.采用FACSort流式细胞仪检测细胞凋亡和细胞周期, 数据采用ModFit软件分析.
1.2.3 蛋白提取与Western印迹杂交: 0.4 μmoL/L GA处理24 h后的细胞用冰PBS洗涤2次, 加入裂解液(见参考文献[8]), 冰上静置10 min, 离心取上清, 采用Braford法测定蛋白质浓度.电泳前加4×SDS缓冲液, 100 ℃水浴10 min, 取40 μg蛋白进行120 g/LSDS-聚丙烯酸胺凝胶电泳, 蛋白分离后, 电泳转至硝酸纤维素膜, 室温封闭2 h, 一抗(1: 1 000) 4 ℃过夜, 二抗室温反应2 h, 采用增强化学发光法(ECL)显影, 暗室压片.胶片结果经英国UVP公司GDS8000型凝胶成像分析系统分析处理数据, 计算灰度值.以β-actin为内参.
统计学处理 采用SAS for Windows6.22软件进行统计学分析, P<0.05有统计学意义
用MTT法测定GA对HepG2细胞的生长抑制作用, 随用药时间的延长抑制作用增强.药物作用24 h抑制率为4.1%, 48 h抑制率为21.7%.
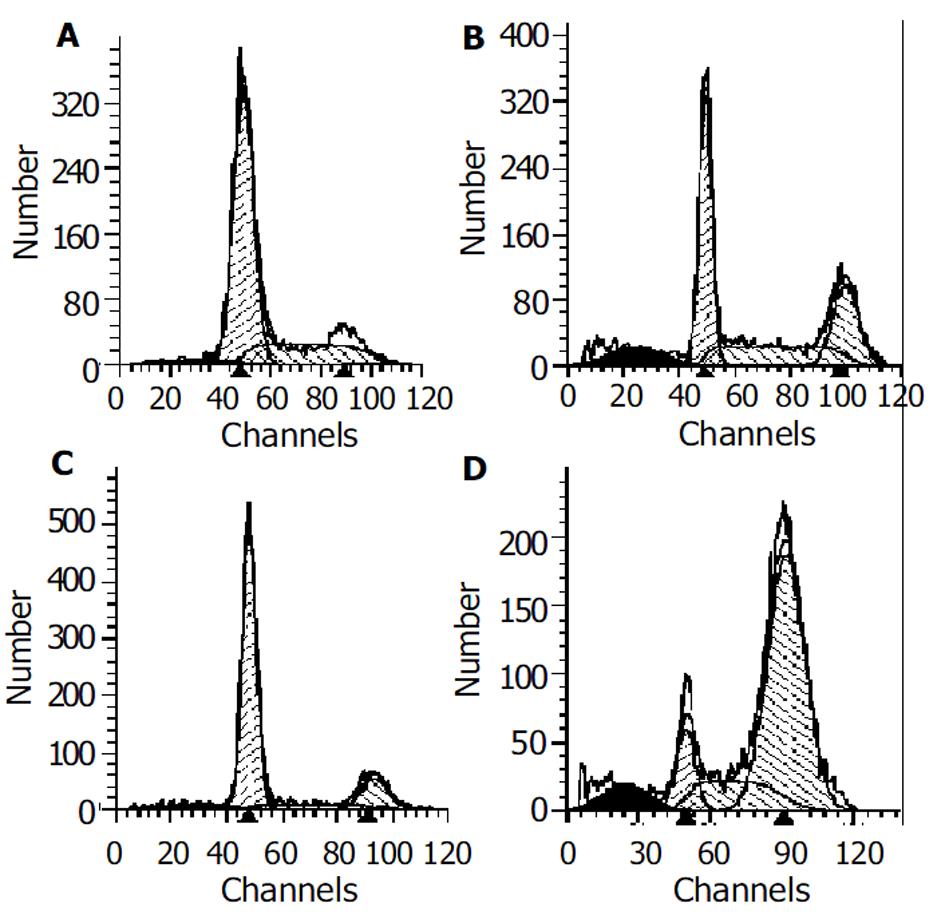
流式细胞术分析表明, 0.4 μmoL/L的GA作用24 h后细胞呈现G2/M期阻滞, 此时G2/M细胞占26.9%, 凋亡率为8.1%; 48 h后G2/M期细胞进一步上升达71.8%, 凋亡率上升为11.4%(图1, 表1).
| 分 组 | 细胞周期(%) | 凋亡率(%) | ||
| G0-G1 | G2/M | S | ||
| 对照组(24 h) | 72.11 | 0.63 | 27.26 | 3.22 |
| 实验组(24 h) | 45.73 | 26.86 | 27.41 | 8.08 |
| 对照组(48 h) | 73.93 | 15.81 | 10.26 | 4.00 |
| 实验组(48 h) | 11.31 | 71.80 | 16.90 | 11.42 |
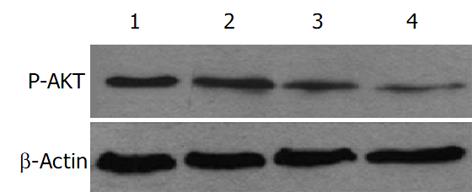
Western印记杂交结果表明, GA作用24及48 h后HepG2细胞中AKT的磷酸化水平下降, 用药时间越长P-AKT水平越低, 分别为对照组的66.0%和34.0%(图2).
分子伴侣是一种与其他蛋白质不稳定构象结合并使之稳定的蛋白, 具有帮助蛋白质分子正确折叠、促进新生肽链成熟、维持蛋白质活性构象的功能, 他们的作用在细胞生命活动中必不可少[9].其中热休克蛋白90(Hsp90)近年来在肿瘤研究领域广受关注.Hsp90的"底物"蛋白质主要是信号转导系统的激酶分子以及一些突变蛋白质如Raf-1[10], ErbB-2[11], Scr [12], Akt[13]以及Bcr-Abl[14], 突变型P53等[15-16], 而这些信号传导分子的过表达或过度激活是肿瘤发生发展中重要的分子事件.Hsp90在肿瘤中表达明显升高, 肝癌组织中Hsp90α的表达与肿瘤的分化程度以及肿瘤大小密切相关[17], Liu et al[18]报道Hsp90反义寡核苷酸转染肝癌SMMC7721细胞可以抑制其生长并增强细胞对常用化疗药的敏感性.因此Hsp90很有可能在肝癌的发生发展中起一定的作用, 干扰Hsp90的功能可能有助于抑制肿瘤的恶性生物学行为.
GA为苯醌安莎霉素类抗生素, 与根壳霉素(radici-col)、除莠霉素(herbimycin A) 一样是由霉菌中分离得到的天然的Hsp90抑制剂[19].其分子中的安莎环可以插入Hsp90肽链N端ADP/ATP结合位点, 抑制Hsp90的弱ATP酶活性, 妨碍其分子伴侣功能的发挥[20-21].Hsp90失活后, 信号传导系统的众多成员将难以稳定存在, 从而产生抑制增生、诱导凋亡的效应[22-23].我们采用MTT法和流式细胞术法检测发现, GA作用后肝癌细胞发生明显的G2/M期阻滞, 并在此基础上产生凋亡增加、生长抑制的效应.目前应用GA衍生物17-AAG治疗癌症的临床试验已取得一定成果 [24], 因此, 通过GA或其衍生物抑制Hsp90对治疗肝癌也有一定帮助.
为进一步研究GA的作用机制, 我们采用Western印记杂交法检测了胞内蛋白激酶B/Akt磷酸化状态的变化. Akt是PI3K/PIP3信号通路的关键下游分子[25], 在受Hsp90调控的众多蛋白质中, 他对于肿瘤的发生发展起重要作用[26].Akt活化后可磷酸化多个下游底物如Bad, Forkhead家簇转录因(FKH-TFs)、NF-κB、mdm2等从而发挥抑制细胞凋亡, 刺激细胞增生的效应[27].Liu et al[28]运用基因芯片技术发现与正常肝组织相比, 肝癌细胞系HLE中Akt的表达上升.还有研究者发现Akt活性的下降与药物引起的肿瘤细胞生长抑制有关[29]. Akt经磷酸化(Ser-473)后被激活, 而磷酸化Akt的水平与Hsp90的功能有很大关系: 一方面, Akt是Hsp90的"底物"蛋白质, 需要Hsp90的辅助方能保持在可被活化的状态; 另一方面, Akt的激活物PDK的稳定存在也受Hsp90调控[30].本研究发现, GA作用后肝癌细胞中磷酸化Akt的水平明显下调, 这是Akt蛋白质的含量下降以及其磷酸化水平降低的共同结果.Okumura et al[31]报道, 被Akt磷酸化后Wee家族的蛋白激酶Myt1失活, 从而改变细胞中cdc25c与Myt1活性的平衡, 促使细胞进入有丝分裂期, 因此Akt被看作是一种G2/M期促进因子.本实验中GA引起的G2/M期阻滞很有可能是因为磷酸化Akt水平的下降所引起的.
总之, Hsp90的功能对肝癌细胞的生长必不可少, 运用GA 抑制Hsp90的功能将导致信号转导系统的关键分子Akt磷酸化水平的下调, 从而产生抑制肝癌细胞生长, 诱导凋亡的效应.这也提示, Hsp90抑制剂在治疗肝癌方面具有应用前景.
编辑: N/A
| 2. | Pratt WB. The hsp90-based chaperone system: involvement in signal transduction from a variety of hormone and growth factor receptors. Proc Soc Exp Biol Med. 1998;217:420-434. [PubMed] [DOI] |
| 3. | Richter K, Buchner J. Hsp90: chaperoning signal transduction. J Cell Physiol. 2001;188:281-290. [PubMed] [DOI] |
| 4. | Cornford PA, Dodson AR, Parsons KF, Desmond AD, Woolfenden A, Fordham M, Neoptolemos JP, Ke Y, Foster CS. Heat shock protein expression independently predicts clinical outcome in prostate cancer. Cancer Res. 2000;60:7099-7105. [PubMed] |
| 5. | Zuo DS, Dai J, Bo AH, Fan J, Xiao XY. Significance of expression of heat shock protein90alpha in human gastric cancer. World J Gastroenterol. 2003;9:2616-2618. [PubMed] |
| 6. | Neckers L, Schulte TW, Mimnaugh E. Geldanamycin as a potential anti-cancer agent: its molecular target and biochemical activity. Invest New Drugs. 1999;17:361-373. [PubMed] [DOI] |
| 7. | Blagosklonny MV. Hsp-90-associated oncoproteins: multiple targets of geldanamycin and its analogs. Leukemia. 2002;16:455-462. [PubMed] [DOI] |
| 8. | Weng LP, Brown JL, Eng C. PTEN coordinates G(1) arrest by down-regulating cyclin D1 via its protein phosphatase activity and up-regulating p27 via its lipid phosphatase activity in a breast cancer model. Hum Mol Genet. 2001;10:599-604. [PubMed] [DOI] |
| 10. | Kim S, Kang J, Hu W, Evers BM, Chung DH. Geldanamycin decreases Raf-1 and Akt levels and induces apoptosis in neuroblastomas. Int J Cancer. 2003;103:352-359. [PubMed] [DOI] |
| 11. | Supino-Rosin L, Yoshimura A, Yarden Y, Elazar Z, Neumann D. Intracellular retention and degradation of the epidermal growth factor receptor, two distinct processes mediated by benzoquinone ansamycins. J Biol Chem. 2000;275:21850-21855. [PubMed] [DOI] |
| 12. | Xu Y, Singer MA, Lindquist S. Maturation of the tyrosine kinase c-src as a kinase and as a substrate depends on the molecular chaperone Hsp90. Proc Natl Acad Sci U S A. 1999;96:109-114. [PubMed] [DOI] |
| 13. | Sato S, Fujita N, Tsuruo T. Modulation of Akt kinase activity by binding to Hsp90. Proc Natl Acad Sci U S A. 2000;97:10832-10837. [PubMed] [DOI] |
| 14. | Nimmanapalli R, O'Bryan E, Bhalla K. Geldanamycin and its analogue 17-allylamino-17-demethoxygeldanamycin lowers Bcr-Abl levels and induces apoptosis and differentiation of Bcr-Abl-positive human leukemic blasts. Cancer Res. 2001;61:1799-1804. [PubMed] |
| 15. | Blagosklonny MV, Toretsky J, Bohen S, Neckers L. Mutant conformation of p53 translated in vitro or in vivo requires functional Hsp90. Proc Natl Acad Sci USA. 1996;93:8379-8383. [DOI] |
| 16. | Workman P. Combinatorial attack on multistep oncogenesis by inhibiting the Hsp90 molecular chaperone. Cancer Lett. 2004;206:149-157. [PubMed] [DOI] |
| 18. | Liu XL, Xiao B, Yu ZC, Guo JC, Zhao QC, Xu L, Shi YQ, Fan DM. Down-regulation of Hsp90 could change cell cycle distribution and increase drug sensitivity of tumor cells. World J Gastroenterol. 1999;5:199-208. [PubMed] [DOI] |
| 19. | Uehara Y. Natural product origins of Hsp90 inhibitors. Curr Cancer Drug Targets. 2003;3:325-330. [PubMed] [DOI] |
| 20. | Workman P. Pharmacogenomics in cancer drug discovery and development: inhibitors of the Hsp90 molecular chaperone. Cancer Detect Prev. 2002;26:405-410. [PubMed] [DOI] |
| 21. | Roe SM, Prodromou C, O'Brien R, Ladbury JE, Piper PW, Pearl LH. Structural basis for inhibition of the Hsp90 molecular chaperone by the antitumor antibiotics radicicol and geldanamycin. J Med Chem. 1999;42:260-266. [PubMed] [DOI] |
| 22. | Stebbins CE, Russo AA, Schneider C, Rosen N, Hartl FU, Pavletich NP. Crystal structure of an Hsp90-geldanamycin complex: targeting of a protein chaperone by an antitumor agent. Cell. 1997;89:239-250. [PubMed] [DOI] |
| 23. | Hostein I, Robertson D, DiStefano F, Workman P, Clarke PA. Inhibition of signal transduction by the Hsp90 inhibitor 17-allylamino-17-demethoxygeldanamycin results in cytostasis and apoptosis. Cancer Res. 2001;61:4003-4009. [PubMed] |
| 24. | Sausville EA, Tomaszewski JE, Ivy P. Clinical development of 17-allylamino, 17-demethoxygeldanamycin. Curr Cancer Drug Targets. 2003;3:377-383. [PubMed] [DOI] |
| 25. | Cantrell DA. Phosphoinositide 3-kinase signalling pathways. J Cell Sci. 2001;114:1439-1445. [PubMed] |
| 26. | Testa JR, Bellacosa A. AKT plays a central role in tumorigenesis. Proc Natl Acad Sci U S A. 2001;98:10983-10985. [PubMed] [DOI] |
| 27. | Li Q, Zhu GD. Targeting serine/threonine protein kinase B/Akt and cell-cycle checkpoint kinases for treating cancer. Curr Top Med Chem. 2002;2:939-971. [PubMed] [DOI] |
| 28. | Liu LX, Liu ZH, Jiang HC, Zhang WH, Qi SY, Hu J, Wang XQ, Wu M. Gene expression profiles of hepatoma cell line HLE. World J Gastroenterol. 2003;9:683-687. [PubMed] [DOI] |
| 29. | Gao S, Yu BP, Li Y, Dong WG, Luo HS. Antiproliferative effect of octreotide on gastric cancer cells mediated by inhibition of Akt/PKB and telomerase. World J Gastroenterol. 2003;9:2362-2365. [PubMed] [DOI] |
| 30. | Solit DB, Basso AD, Olshen AB, Scher HI, Rosen N. Inhibition of heat shock protein 90 function down-regulates Akt kinase and sensitizes tumors to Taxol. Cancer Res. 2003;63:2139-2144. [PubMed] |
| 31. | Okumura E, Fukuhara T, Yoshida H, Hanada Si S, Kozutsumi R, Mori M, Tachibana K, Kishimoto T. Akt inhibits Myt1 in the signalling pathway that leads to meiotic G2/M-phase transition. Nat Cell Biol. 2002;4:111-116. [PubMed] [DOI] |