修回日期: 2004-03-25
接受日期: 2004-04-05
在线出版日期: 2004-08-15
目的: 探讨TGIF(TG interacting factor)蛋白在食管癌中的表达水平及其临床病理意义.
方法: 应用免疫组织化学SP法, 对32例食管癌的活检和手术切除标本进行抗人TGIF抗体免疫组织化学染色, 检测癌组织和癌旁组织TGIF的表达, 并分析TGIF的表达与食管癌临床病理特征之间的关系.
结果: TGIF在食管黏膜鳞状上皮和食管癌组织中阳性率分别为60.0%和71.2%, 但后者以中度-强阳性为主. TGIF蛋白在食管癌组织中的表达水平明显高于正常食管黏膜鳞状上皮(P = 0.036), 但与性别、肿瘤的分化、浸润深度及淋巴结的转移无关.
结论: TGIF通过抑制TGF-β和视黄醛促进食管癌的发生与发展.
引文著录: 胡忠良, 文继舫, 郑晖, 傅春燕. 食管癌组织TGIF表达的意义. 世界华人消化杂志 2004; 12(8): 1766-1768
Revised: March 25, 2004
Accepted: April 5, 2004
Published online: August 15, 2004
AIM: To investigate the expression level of TG interacting factor (TGIF) in esophageal carcinomas, and the correlation between TGIF and clinicopathological features of esophageal carcinoma.
METHODS: A total of 32 specimens of esophageal carcinoma were investigated by immunohistochemical staining with a polyclonal antibody against TGIF. The expression of TGIF in esophageal carcinoma was detected and the relationship between the expression of TGIF and clinico-pathological features of esophageal carcinoma was analyzed.
RESULTS: The positive rates of TGIF in normal esophageal squamous epithelium and esophageal carcinoma were 60% and 71.2%, respectively, but the latter had stronger intensity staining. The expression level of TGIF in esophageal carcinoma was higher than that in normal esophageal squamous epithelium (P = 0.036), but had no correlation with gender, depth of invasion, tumor grade and lymph node metastasis.
CONCLUSION: TGIF is a possible oncogene, and it may promote the progression of esophageal carcinomas via inhibiting both the TGF-β and retinoid signaling pathways.
- Citation: Hu ZL, Wen JF, Zhen H, Fu CY. Expression of TGIF in esophageal carcinoma and its clinicopathological features. Shijie Huaren Xiaohua Zazhi 2004; 12(8): 1766-1768
- URL: https://www.wjgnet.com/1009-3079/full/v12/i8/1766.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v12.i8.1766
食管癌是一种严重威胁我国人民生命健康的常见肿瘤, 但其确切的发病机制尚不清楚[1-4]. 许多肿瘤的发生与TGF-β相关, 多数肿瘤丧失了TGF-β介导的生长抑制作用[5]. TGIF(TG-interacting factor, TGIF)能通过多种方式抑制TGF-β信号通路[6-9]. MAPK通路可磷酸化TGIF使其半衰期延长, 从而引起TGIF蛋白浓度升高[7]. TGIF功能的增强可能抑制TGF-β对细胞的负性调节作用. 为探讨TGIF在肿瘤发生中的作用, 我们应用免疫组织化学方法, 分析TGIF蛋白在食管癌中的表达水平及其临床病理意义.
中南大学湘雅医院病理科2000-2001年食管癌石蜡包埋组织32例. 男29例, 女4例, 年龄37-75岁(平均59±9岁); 高分化12例, 中分化13例, 低分化7例. 所有标本经40 g/L甲醛固定, 常规石蜡包埋, 临床资料和病理诊断准确无误. 并分别选用20例食管癌的远断端(距肿块边缘均在5 cm以上)作为对照组. 全部蜡块均切成4 μm厚, 60 ℃干烤4 h, 进行免疫组织化学染色. 多克隆性抗TGIF抗体(羊)sc-9826为Santa Cruz Biotechnology, Inc. 产品, 工作浓度为1: 100; SP-Streptavidin Peroxidase kit购自北京中山生物技术有限公司.
辣根过氧化酶标记的SP 法. 即: 石蜡切片常规脱蜡至水, 30 mL/L 过氧化氢-甲醇封闭5 min, PBS(pH7.2)漂洗后置EDTA中, 微波炉抗原修复10 min, PBS 漂洗后依次滴加一抗37 ℃ 30 min并 4 ℃过夜, 二抗37 ℃ 30 min, 辣根酶标记的链霉卵白素工作液30 min 及显色底物, 于显微镜监视下显色, 自来水充分冲洗终止反应, 苏木素复染, 脱水, 透明, 封片(以PBS 代替一抗为阴性对照). TGIF阳性表达以细胞质或细胞核出现明显的棕黄色颗粒为准. 免疫组织化学染色阳性细胞分4级: 无棕黄色颗粒(-); 轻度棕黄色颗粒(+); 中度棕黄色颗粒(++); 重度棕黄色颗粒(+++).
统计学处理 等级资料的秩和检验(Krukal Wallis'of Nemenyis'), 检验水准: α = 0.05.
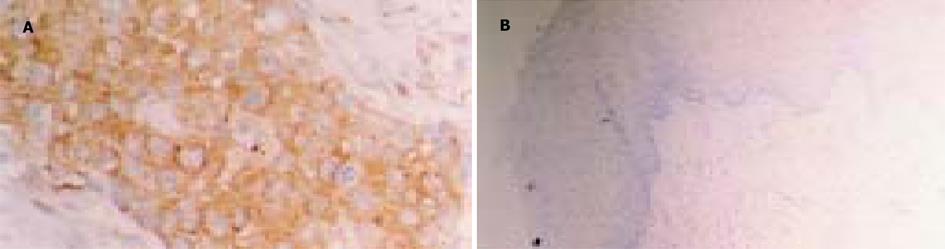
TGIF蛋白的表达主要定位于胞质, 多位于核周, 部分为核阳性. 他在部分食管黏膜鳞状上皮中表达(图1), 阳性率60%, 但多为轻度阳性. 食管癌组织TGIF蛋白的阳性率为71.2%, 阳性程度以中度-强阳性为主. 统计学分析表明TGIF蛋白在食管癌组织中的表达水平明显高于正常食管黏膜鳞状上皮(图1)(P = 0.036), 但与性别、肿瘤的分化、浸润深度及淋巴结的转移无关(表1)(P>0.05).
TGIF基因定位于18p11, 属于同型结构域TALE家族. 他参与多种生物的发育过程, TGIF的基因突变致功能失活后, 能引起人前脑无裂畸形[10]. TGIF表达缺失型雄性果蝇尽管外观正常, 但丧失了生殖能力[11-12]. 此外, TGIF可抑制TGF-β和视黄醛等细胞信号通路, 而TGF-β和视黄醛能抑制上皮细胞生长, 诱导细胞的分化和凋亡. TGIF在肿瘤发生中的作用尚不明确, Voorter et al[13]通过比较基因组杂交发现在膀胱移行细胞癌中18p11存在基因扩增, 人类TGIF蛋白的过表达能使酵母细胞通过G1期细胞周期阻滞点[14], Luo et al[15]发现在卵巢癌患者的血清中存在针对TGIF的抗体. 我们的研究结果表明TGIF蛋白在食管癌组织中有过表达, Nakakuki et al[16]发现在食管癌细胞系中有TGIF基因的扩增, TGIF有可能通过抑制TGF-β和视黄醛这两条信号通路而促进食管癌的发生与发展, TGIF在食管癌中可能起癌基因的作用. 但TGIF的表达与性别、肿瘤的分化、浸润深度及淋巴结的转移无关.
TGIF为核蛋白, 但我们的研究却表明阳性信号主要定位于胞质, 散在分布于胞核, 这可能是由于TGIF蛋白的合成必须在胞质中进行, 胞质中积聚大量TGIF前体蛋白的缘故. 总之, TGIF在肿瘤发生中的作用有待进一步的研究.
编辑: N/A
| 1. | 王 立峰, 张 丽红, 刘 明, 张 伟, 王 吾如, 王 洪平, 刘 伯齐, 金 玉生, 靳 玉兰, 韩 志楷. 食管癌及 其癌前病变组织p16蛋白表达的研究. 世界华人消化杂志. 2003;11:90-91. [DOI] |
| 5. | Wieser R. The transforming growth factor-beta signaling pathway in tumorigenesis. Curr Opin Oncol. 2001;13:70-77. [PubMed] [DOI] |
| 6. | Wotton D, Knoepfler PS, Laherty CD, Eisenman RN, Massagué J. The Smad transcriptional corepressor TGIF recruits mSin3. Cell Growth Differ. 2001;12:457-463. [PubMed] |
| 7. | Lo RS, Wotton D, Massagué J. Epidermal growth factor signaling via Ras controls the Smad transcriptional co-repressor TGIF. EMBO J. 2001;20:128-136. [PubMed] [DOI] |
| 8. | Pessah M, Prunier C, Marais J, Ferrand N, Mazars A, Lallemand F, Gauthier JM, Atfi A. c-Jun interacts with the corepressor TG-interacting factor (TGIF) to suppress Smad2 transcriptional activity. Proc Natl Acad Sci U S A. 2001;98:6198-6203. [PubMed] [DOI] |
| 9. | Chen F, Ogawa K, Nagarajan RP, Zhang M, Kuang C, Chen Y. Regulation of TG-interacting factor by transforming growth factor-beta. Biochem J. 2003;371:257-263. [PubMed] [DOI] |
| 10. | Gripp KW, Wotton D, Edwards MC, Roessler E, Ades L, Meinecke P, Richieri-Costa A, Zackai EH, Massagué J, Muenke M. Mutations in TGIF cause holoprosencephaly and link NODAL signalling to human neural axis determination. Nat Genet. 2000;25:205-208. [PubMed] [DOI] |
| 11. | Wang Z, Mann RS. Requirement for two nearly identical TGIF-related homeobox genes in Drosophila spermatogenesis. Development. 2003;130:2853-2865. [PubMed] [DOI] |
| 12. | Ayyar S, Jiang J, Collu A, White-Cooper H, White RA. Drosophila TGIF is essential for developmentally regulated transcription in spermatogenesis. Development. 2003;130:2841-2852. [PubMed] [DOI] |
| 13. | Voorter C, Joos S, Bringuier PP, Vallinga M, Poddighe P, Schalken J, du Manoir S, Ramaekers F, Lichter P, Hopman A. Detection of chromosomal imbalances in transitional cell carcinoma of the bladder by comparative genomic hybridization. Am J Pathol. 1995;146:1341-1354. [PubMed] |
| 14. | Edwards MC, Liegeois N, Horecka J, DePinho RA, Sprague GF Jr, Tyers M, Elledge SJ. Human CPR (cell cycle progression restoration) genes impart a Far- phenotype on yeast cells. Genetics. 1997;147:1063-1076. [PubMed] |
| 15. | Luo LY, Herrera I, Soosaipillai A, Diamandis EP. Identification of heat shock protein 90 and other proteins as tumour antigens by serological screening of an ovarian carcinoma expression library. Br J Cancer. 2002;87:339-343. [PubMed] [DOI] |
| 16. | Nakakuki K, Imoto I, Pimkhaokham A, Fukuda Y, Shimada Y, Imamura M, Amagasa T, Inazawa J. Novel targets for the 18p11.3 amplification frequently observed in esophageal squamous cell carcinomas. Carcinogenesis. 2002;23:19-24. [PubMed] [DOI] |