修回日期: 2004-04-01
接受日期: 2004-04-15
在线出版日期: 2004-07-15
目的: 探讨醛固酮受体拮抗剂螺内酯对TGFβ1和TIMP-1表达的作用, 评价螺内酯的抗纤维化作用.
方法: ♂SD大鼠34只, 随机分为3组. 肝硬化模型组: 400 mL/L CCl4油3 mL/kg, sc, 2次/wk; 螺内酯组: CCl4油注射的同时予螺内酯20 mg/(kg/d)灌胃; 正常对照组: 正常饮食、饮水. 光镜下动态观察组织学改变, RT-PCR检测肝组织中TGFβ1和TIMP-1的表达.
结果: 10 wk时, 螺内酯组肝纤维化级别显著低于肝纤维化模型组(P<0.05); 但在13 wk, 螺内酯组与肝纤维化模型组相比无显著性差异. 肝纤维化时TGFβ1和TIMP-1 mRNA水平与正常组相比显著升高(P<0.05). 在10 wk时, 螺内酯组TGFβ1 TIMP-1 mRNA水平与肝纤维化模型组相比有下降的趋势, 但无显著性差异.
结论: TGFβ1和TIMP-1在肝纤维化时表达显著增加, 螺内酯对肝纤维化有一定程度的抑制作用, 但对TGFβ1和TIMP-1 mRNA的表达作用不明显.
引文著录: 黄欣, 朱纲, 王磊. 螺内酯对大鼠肝纤维化及TGFβ1 TIMP-1表达的作用. 世界华人消化杂志 2004; 12(7): 1604-1607
Revised: April 1, 2004
Accepted: April 15, 2004
Published online: July 15, 2004
AIM: To identify the effects of spironolactone on expressions of transforming growth factor β1 and tissue inhibitor of metalloproteinase-1 and to evaluate the curative effect of spironolactone.
METHODS: Thirty four male SD rats were randomly divided into 3 groups: Hepatic fibrosis model group: the rats were injected with 400 mL/L CCl4 3 mL/kg subcutaneously two times a week; Spironolactone group: the rats were injected with 400 mL/L CCl4 3 mL/kg subcutaneously two times a week; Spironolactone equivalent to 20 mg/kg per day was given intragastrically; Normal control group: normal chow. Hepatic tissue was observed with light microscopy to compare histological alterations dynamically. The expression of transforming growth factor β1 and tissue inhibitor of metalloproteinase-1 was detected by RT-PCR.
RESULTS: The grades of fibrosis in spironolactone group were less than those in hepatic fibrosis model group at the end of the week 10 (P < 0.05). However, at the end of week 13, there was no significant difference between the two groups. The expression of TGFβ1 and TIMP-1 mRNA was up-regulated when fibrogenesis occurred (P < 0.05). Although at the end of week 10 there was an inclination that the levels of TGFβ1 and TIMP-1 mRNA in spironolactone group were less than those in hepatic fibrosis model group, there was no significant difference between two groups.
CONCLUSION: The expression of TGFβ1 and TIMP-1 mRNA is up-regulated significantly when fibrogenesis occurs. Spironolactone may have a fibrogenesis-inhibiting effect on CCl4 -induced hepatic fibrosis in some degree. Spironolactone has no significant effect on the expression of TGFβ1 and TIMP-1 mRNA
- Citation: Huang X, Zhu G, Wang L. Effects of spironolactone on rat liver fibrosis and expression of transforming growth factor β1 and tissue inhibitor of metalloproteinase-1. Shijie Huaren Xiaohua Zazhi 2004; 12(7): 1604-1607
- URL: https://www.wjgnet.com/1009-3079/full/v12/i7/1604.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v12.i7.1604
肝损伤时释放转化生长因子1(TGFβ1)等可直接激活肝星形细胞(HSC), 而活化的HSC又可自分泌TGFβ1进一步发挥作用. 在肝纤维化形成过程中, 除了胶原的合成增多外, 胶原降解减少也是一个重要因素. I, Ⅲ型胶原主要由基质金属蛋白酶-1(MMP-1)降解, 金属蛋白酶组织抑制因子-1(TIMP-1)可与MMP-1特异性结合而抑制其活性. 肝纤维化时TIMP-1的表达显著增加[1-14]. TGFβ1不仅能促进胶原的合成, 而且可增加TIMP-1表达[15], 因而是肝纤维化过程中关键的细胞因子之一. 近年来的研究表明, 除肾上腺外, 心脏、血管、脑、肝、肺、肾等均能合成醛固酮, 并且在器官纤维化中具有重要作用.醛固酮受体拮抗剂螺内酯能有效减轻心脏和肺的纤维化程度[16-24]. 为了探讨螺内酯对肝纤维化的作用及其机制, 我们研究了螺内酯对CCl4诱导的大鼠肝纤维化程度以及肝组织TGFβ1和TIMP-1表达的作用.
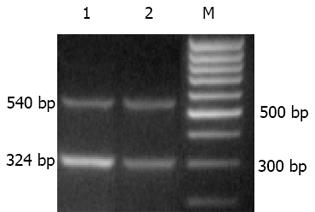
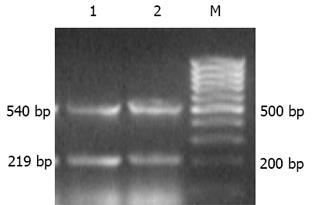
♂SD大鼠34只, 清洁级, 质量250-300 g. 购自上海中英合资必凯公司. 随机分为3组. 肝纤维化模型组(15只): 400 mL/L CCl4(精制橄榄油配制)sc, 3 mL/kg, 2次/wk; 螺内酯组(13只): 在CCl4诱导肝纤维化的同时, 予螺内酯(溶于蒸馏水中)20 mg/(kg/d)灌胃; 正常对照组(6只): 正常饮食、饮水. 分别于造模后10, 13 wk末处死肝纤维化模型组及螺内酯组大鼠各6只, 正常对照组6只大鼠全部于13 wk末处死. 取肝脏右叶组织, 中性甲醛固定, 石蜡包埋, 切片, HE染色后由同一病理专科医师按文献[25]标准进行肝纤维化分级. 0级正常, 6级为早期肝硬化. RT-PCR试剂盒、dNTP、TaqDNA聚合酶等均为大连Takara公司产品. 所用引物由上海生工生物工程技术服务有限公司合成. TGFβ1引物序列为上游5'CTTCAGCTCCACAGAGAAGAACTGC3', 下游5'CACGATCATGTTGGACAACTGCTCC 3', 扩增长度324 bp; TIMP-1引物序列为上游: 5'GACCTGGTCATAAGGGCTAAA 3', 下游: 5'GCCCGTGATGAGAAACTCTTCACT 3', 扩增长度219 bp; -actin引物序列为上游: 5'GTGGGCCGCTCTAGGCACCAA 3', 下游: 5'CTCTTTGATGTCACGCACGATTTC 3', 扩增长度540 bp.
大鼠肝组织总RNA抽提, 操作步骤按说明书进行. 剪取50-100 mg肝组织加入Trizol试剂(美国Gibcol 公 司)溶液1 mL, 冰浴中彻底匀浆, 在匀浆后的样本中加入氯仿0.4 mL, 混匀, 冰置15 min后4 ℃12 000 g离心15 min. 取上清液, 加入等体积的异丙醇, 混匀, 放置10 min后4 ℃ 12 000 g离心10 min, 弃上清液, 加入75%乙醇1 mL, 混匀, 4 ℃ 7 500 g离心5 min, 弃去上清液. 紫外分光光度计于260 nm波长测定RNA含量. 然后按逆转录反应试剂盒操作程序进行逆转录合成cDNA. 取总RNA 4.5 g, 逆转录酶1 L, dNTP混合物(10 mmoL/L)2 L, olig dT-Adaptor 引物1 L, 10×PCR缓冲液2 L, MgCl2(25 mmol/L)4 L, RNA酶抑制剂0.5 L, 加无RNA酶水至总体积20 L. 反应条件: 30 ℃ 10 min, 42 ℃ 60 min, 99 ℃ 5 min, 5 ℃ 5 min, 合成cDNA. PCR扩增反应体系: dNTP混合物(10 mmoL/L) 0.5 L, 10×PCR缓冲液2 L, MgCl2(25 mmoL/L)1.2 L, Taq酶(5 MU/L)0.25 L, 上下游引物(20 mmol/L)各1 L, 逆转录cDNA产物2 L, 其余为无RNA酶水, 反应总体积20 L. TGF-β1扩增参数: 首先94 ℃预变性5 min; 然后94 ℃变性30 s, 61 ℃退火1 min, 72 ℃延伸45 s, 共35个循环, 最后72 ℃10 min以充分延伸. TIMP-1扩增参数: 首先94 ℃预变性5 min; 之后94 ℃变性 1 min; 退火56 ℃ 2 min; 延伸72 ℃1.5 min; 共35个循环, 最后72 ℃, 10 min, 终止反应. 分别以相同条件扩增鼠-actin, 作为内参照. 取15 L CR产物于15 g/L的琼脂糖凝胶电泳. 凝胶图像分析系统(上海天能科技有限公司)检测DNA条带平均灰度值.
统计学处理 等级资料采用Ridit检验; 计量资料用均数±标准差(mean±SD)表示, 两组均数比较用t检验.
肝纤维化模型组大鼠毛色暗淡, 厌食, 消瘦, 尿色深黄, 死亡3只. 螺内酯组大鼠毛色较好, 食欲尚可, 质量较重, 死亡1只. 正常组大鼠一般情况良好, 无死亡.
肝纤维化模型组10 wk时汇管区纤维组织增生, 伴明显的P-P桥(汇管区间的桥状纤维化)或P-C桥(汇管区-中央静脉间纤维组织增生)形成, 或可见结节形成. 螺内酯组肝纤维化程度低于模型组, 两组肝纤维化分级有显著性差异(P<0.05, 表1). 13 wk时, 肝纤维化模型组和螺内酯组均纤维组织增生明显, 肝纤维化分级两组无显著性差异 (P>0.05). 但螺内酯组仅有1只大鼠发生肝硬化, 而肝纤维化模型组则有3只. 肝硬化发生率有下降的趋势(表1, 图1.
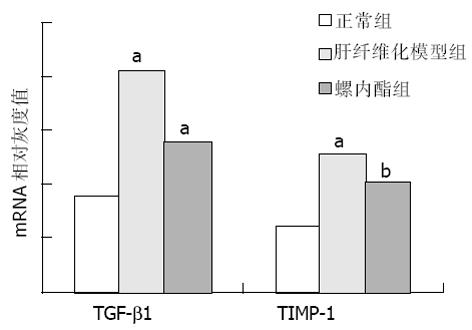
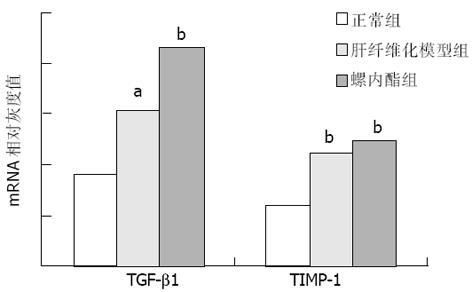
肝纤维化时TGFβ1和TIMP-1 mRNA水平与正常组相比显著升高(P<0.05). 在10 wk时, 螺内酯组TGFβ1, TIMP-1 mRNA水平与肝纤维化模型组相比有下降的趋势, 但10及13 wk两组间表达均无显著性差异(表2; 图2-4).
在实验性大鼠肝纤维化的发展过程中, 肝脏编码醛固酮合成酶的CYP11B2基因表达明显上调. 由于CYP11B2基因编码的是醛固酮合成后期阶段的关键酶, 因此, 提示醛固酮合成增加与肝纤维化的形成可能有密切关系. 原位杂交显示CYP11B2定位于肝星状细胞(HSC)内[26]. 在体外用无血清培养基培养原代HSC和HSC-T6细胞株, 同样能检测到CYP11B2mRNA的表达和醛固酮的合成[27]. 说明HSC表达和合成醛固酮. 进一步研究发现, 醛固酮可促进体外培养HSC-T6的Ⅰ, Ⅲ型前胶原mRNA的表达与胶原合成, 并对TIMP-1mRNA的表达也具有促进作用[28-29].
螺内酯作为醛固酮的拮抗剂, 可通过与醛固酮竞争性地结合受体, 以抑制醛固酮的作用.本结果显示, 在10 wk时, 螺内酯组大鼠的肝纤维化程度较模型组明显减轻, 具有统计学意义(P<0.05). 至13 wk时, 模型组有3只大鼠发生肝硬化, 而螺内酯组仅有1只. 从肝纤维化分级来看, 螺内酯组大多处于2级(1只)和3级(3只), 而模型组为4级(1只)和5级(2只). 虽然统计学处理二组之间无显著差异, 但螺内酯组肝硬化的发生率和肝纤维化程度均较模型组有下降趋势. 这可能与实验大鼠数量较少有关, 增加样本量有可能使这一趋势更加明确. 这表明螺内酯对CCl4诱导的肝纤维化, 特别是在早期具有一定的预防作用, 与文献[26]报道的一致.推测在肝纤维化早期, 肝组织中醛固酮的表达量可能相对较少, 因此螺内酯的拮抗作用较为明显, 以后随着病程发展, 醛固酮含量增多, 螺内酯的竞争性抑制作用被削弱. 研究发现[30], 随着肝纤维化的进展, 醛固酮的生成增加, 对此提供了证据.此外, 肝纤维化是一个由多种因素共同参与、相互作用的复杂过程, 醛固酮只是其中的因素之一. TGFβ1, TIMP-1在肝纤维化时表达显著增加, 但10及13 wk螺内酯组和模型组间表达均无显著性差异. 提示螺内酯对肝纤维化的抑制作用有可能不是通过直接抑制TGFβ1、TIMP-1的表达实现的.另外, 我们仅对TGFβ1, TIMP-1的转录水平进行了研究, 还没有观察两个分子在转录后蛋白质水平有无变化. 关于醛固酮对肝纤维化的作用及其机制, 目前尚未完全明了, 有待进一步深入研究.
编辑: N/A
| 1. | Nie QH, Cheng YQ, Xie YM, Zhou YX, Bai XG, Cao YZ. Methodologic research on TIMP-1, TIMP-2 detection as a new diagnostic index for hepatic fibrosis and its significance. World J Gastroenterol. 2002;8:282-287. [PubMed] [DOI] |
| 2. | Nie QH, Duan GR, Luo XD, Xie YM, Luo H, Zhou YX, Pan BR. Expression of TIMP-1 and TIMP-2 in rats with hepatic fibrosis. World J Gastroenterol. 2004;10:86-90. [PubMed] [DOI] |
| 3. | Zhang BB, Cai WM, Weng HL, Hu ZR, Lu J, Zheng M, Liu RH. Diagnostic value of platelet derived growth factor-BB, transforming growth factor-beta1, matrix metalloproteinase-1, and tissue inhibitor of matrix metalloproteinase-1 in serum and peripheral blood mononuclear cells for hepatic fibrosis. World J Gastroenterol. 2003;9:2490-2496. [PubMed] [DOI] |
| 4. | Nie QH, Cheng YQ, Xie YM, Zhou YX, Cao YZ. Inhibiting effect of antisense oligonucleotides phosphorthioate on gene expression of TIMP-1 in rat liver fibrosis. World J Gastroenterol. 2001;7:363-369. [PubMed] [DOI] |
| 5. | García-Tevijano ER, Berasain C, Rodríguez JA, Corrales FJ, Arias R, Martín-Duce A, Caballería J, Mato JM, Avila MA. Hyperhomocysteinemia in liver cirrhosis: mechanisms and role in vascular and hepatic fibrosis. Hypertension. 2001;38:1217-1221. [PubMed] [DOI] |
| 6. | Arthur MJ. Fibrogenesis II. Metalloproteinases and their inhibitors in liver fibrosis. Am J Physiol Gastrointest Liver Physiol. 2000;279:G245-G249. [PubMed] |
| 7. | Kossakowska AE, Edwards DR, Lee SS, Urbanski LS, Stabbler AL, Zhang CL, Phillips BW, Zhang Y, Urbanski SJ. Altered balance between matrix metalloproteinases and their inhibitors in experimental biliary fibrosis. Am J Pathol. 1998;153:1895-1902. [PubMed] [DOI] |
| 8. | Iredale JP, Benyon RC, Pickering J, McCullen M, Northrop M, Pawley S, Hovell C, Arthur MJ. Mechanisms of spontaneous resolution of rat liver fibrosis. Hepatic stellate cell apoptosis and reduced hepatic expression of metalloproteinase inhibitors. J Clin Invest. 1998;102:538-549. [PubMed] [DOI] |
| 9. | Böker KH, Pehle B, Steinmetz C, Breitenstein K, Bahr M, Lichtinghagen R. Tissue inhibitors of metalloproteinases in liver and serum/plasma in chronic active hepatitis C and HCV-induced cirrhosis. Hepatogastroenterology. 2000;47:812-819. [PubMed] |
| 10. | Boeker KH, Haberkorn CI, Michels D, Flemming P, Manns MP, Lichtinghagen R. Diagnostic potential of circulating TIMP-1 and MMP-2 as markers of liver fibrosis in patients with chronic hepatitis C. Clin Chim Acta. 2002;316:71-81. [PubMed] [DOI] |
| 11. | Melgert BN, Olinga P, Van Der Laan JM, Weert B, Cho J, Schuppan D, Groothuis GM, Meijer DK, Poelstra K. Targeting dexamethasone to Kupffer cells: effects on liver inflammation and fibrosis in rats. Hepatology. 2001;34:719-728. [PubMed] [DOI] |
| 12. | Knittel T, Mehde M, Grundmann A, Saile B, Scharf JG, Ramadori G. Expression of matrix metalloproteinases and their inhibitors during hepatic tissue repair in the rat. Histochem Cell Biol. 2000;113:443-453. [PubMed] |
| 13. | Murawaki Y, Ikuta Y, Kawasaki H. Clinical usefulness of serum tissue inhibitor of metalloproteinases (TIMP)-2 assay in patients with chronic liver disease in comparison with serum TIMP-1. Clin Chim Acta. 1999;281:109-120. [PubMed] [DOI] |
| 14. | Lichtinghagen R, Breitenstein K, Arndt B, Kühbacher T, Böker KH. Comparison of matrix metalloproteinase expression in normal and cirrhotic human liver. Virchows Arch. 1998;432:153-158. [PubMed] [DOI] |
| 15. | Herbst H, Wege T, Milani S, Pellegrini G, Orzechowski HD, Bechstein WO, Neuhaus P, Gressner AM, Schuppan D. Tissue inhibitor of metalloproteinase-1 and -2 RNA expression in rat and human liver fibrosis. Am J Pathol. 1997;150:1647-1659. [PubMed] |
| 16. | Hayashi M, Tsutamoto T, Wada A, Tsutsui T, Ishii C, Ohno K, Fujii M, Taniguchi A, Hamatani T, Nozato Y. Immediate administration of mineralocorticoid receptor antagonist spironolactone prevents post-infarct left ventricular remodeling associated with suppression of a marker of myocardial collagen synthesis in patients with first anterior acute myocardial infarction. Circulation. 2003;107:2559-2565. [PubMed] [DOI] |
| 17. | Mandarim-de-Lacerda CA, Pereira LM. The effects of spironolactone monotherapy on blood pressure and myocardial remodeling in spontaneously hypertensive rats: a stereological study. J Biomed Sci. 2003;10:50-57. [PubMed] [DOI] |
| 18. | Pereira LM, Mandarim-de-Lacerda CA. Myocardial changes after spironolactone in spontaneous hypertensive rats. A laser scanning confocal microscopy study. J Cell Mol Med. 2002;6:49-57. [DOI] |
| 19. | Gallego M, Espiña L, Vegas L, Echevarria E, Iriarte MM, Casis O. Spironolactone and captopril attenuates isoproterenol-induced cardiac remodelling in rats. Pharmacol Res. 2001;44:311-315. [PubMed] [DOI] |
| 20. | Miric G, Dallemagne C, Endre Z, Margolin S, Taylor SM, Brown L. Reversal of cardiac and renal fibrosis by pirfenidone and spironolactone in streptozotocin-diabetic rats. Br J Pharmacol. 2001;133:687-694. [PubMed] [DOI] |
| 21. | Tsutamoto T, Wada A, Maeda K, Mabuchi N, Hayashi M, Tsutsui T, Ohnishi M, Sawaki M, Fujii M, Matsumoto T. Effect of spironolactone on plasma brain natriuretic peptide and left ventricular remodeling in patients with congestive heart failure. J Am Coll Cardiol. 2001;37:1228-1233. [PubMed] [DOI] |
| 22. | Zannad F, Alla F, Dousset B, Perez A, Pitt B. Limitation of excessive extracellular matrix turnover may contribute to survival benefit of spironolactone therapy in patients with congestive heart failure: insights from the randomized aldactone evaluation study (RALES). Rales Investigators. Circulation. 2000;102:2700-2706. [PubMed] [DOI] |
| 23. | Sato A, Suzuki Y, Saruta T. Effects of spironolactone and angiotensin-converting enzyme inhibitor on left ventricular hypertrophy in patients with essential hypertension. Hypertens Res. 1999;22:17-22. [PubMed] [DOI] |
| 25. | Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, Denk H, Desmet V, Korb G, MacSween RNM. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696-699. [DOI] |