修回日期: 2004-03-01
接受日期: 2004-04-05
在线出版日期: 2004-07-15
目的: 研究小檗碱对大鼠肝细胞延迟外向钾电流(IK)和钙释放激活钙电流(ICRAC)的影响.
方法: 用酶消化法分离大鼠肝细胞, 应用全细胞膜片钳技术, 研究小檗碱对大鼠肝细胞延迟外向钾电流(IK)和钙释放激活钙电流(ICRAC)的影响.
结果: 小檗碱1-300 moL/L浓度依赖性地抑制IK, 其 EC50值为39±5 moL/L, nH为0.82±0.05, 30 moL/L小檗碱在膜电位为+60 ~+140 mV显著降低IK幅值, I-V曲线下移(n = 8, P<0.05 or P<0.01 vs control). 小檗碱1-300 moL/L亦浓度依赖性地抑制ICRAC, EC50值为47±11 moL/L, nH为0.71±0.09. 小檗碱30 mol/L使各膜电位下ICRAC幅值降低, 对内向电流和外向电流均明显减小, 尤其减小膜电位为-100 ~-80 mV时的ICRAC幅值(n = 8, P<0.05 vs control).
结论: 小檗碱对大鼠肝细胞钾电流和钙电流均有抑制作用, 该作用可能与其肝保护作用有关.
引文著录: 王芳, 程岚, 赵刚, 周红义, 姚伟星. 小檗碱对大鼠肝细胞钾电流和钙电流的影响. 世界华人消化杂志 2004; 12(7): 1600-1603
Revised: March 1, 2004
Accepted: April 5, 2004
Published online: July 15, 2004
AIM: To study the effects of berberine on ion channels in isolated rat hepatocytes.
METHODS: Tight-seal whole-cell patch-clamp techniques were performed to investigate the effects of berberine on the delayed outward potassium currents (IK) and Ca2+ release-activated Ca2+ current (ICRAC) in enzymatically isolated rat hepatocytes.
RESULTS: Berberine 1-300 moL/L reduced IK in a concentration-dependent manner with EC50 of 39±5 moL/L and nH of 0.82±0.05 (n = 8). When the bath solution was changed to tetraethylammonium (TEA) 8 mmol/L, IK was inhibited. Berberine 30 mol/L reduced IK at all membrane potentials examined, especially at potentials positive to +60 mV (n = 8, P < 0.05 or P < 0.01 vs control). Berberine 1-300 moL/L also inhibited ICRAC in a concentration-dependent manner. The fitting parameters were as follows: EC50 = 47±11 moL/L, nH = 0.71±0.09 (n = 8). The peak value of ICRAC in the I-V relationship was decreased by berberine 30 mol/L at potential negative to -80 mV (n = 8, P < 0.05 vs control). But the reverse potential of ICRAC occurred at voltage = 0 mV in all cells.
CONCLUSION: Berberine has inhibitory effects on potassium and calcium currents in isolated rat hepatocytes, which may be involved in the hepatoprotective effect.
- Citation: Wang F, Cheng L, Zhao G, Zhou HY, Yao WX. Effects of berberine on potassium and calcium currents in isolated rat hepatocytes. Shijie Huaren Xiaohua Zazhi 2004; 12(7): 1600-1603
- URL: https://www.wjgnet.com/1009-3079/full/v12/i7/1600.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v12.i7.1600
小檗碱(berberine, Ber)是从毛茛科黄连属(Coptis chinensis franch)根状茎中提取的异喹啉类生物碱. Ber可浓度依赖性地抑制豚鼠心室肌细胞延迟整流钾电流和L-型钙电流[1-2]. Hwang et al[3]报道Ber可拮抗叔丁基过氧化氢(tert-butyl hydroperoxide, t -BHP)诱导的大鼠氧化应激性肝损伤, 降低血清转氨酶及碱性磷酸酶, 增加肝血流量, 增强肝细胞再生能力, 促进肝损伤的修复过程. Ber的肝脏保护作用是否与其对肝细胞离子通道的作用有关, 目前尚未见相关研究报道. 我们采用全细胞膜片钳技术, 研究Ber对大鼠肝细胞延迟外向钾电流(IK)和钙释放激活钙电流(ICRAC)的影响, 以期从通道水平阐明其肝保护作用的机制.
D-Hanks液成分为(mmoL/L): NaCl 137, KCl 5.4, NaH2PO4 0.5, Na2HPO4 4.16, Glucose 5.5, 用NaOH调节pH值至7.3. KB液成分为(mmoL/L): Glutamic acid 70, KCl 130, taurine 15, K2HPO4 10, MgCl2 0.5, HEPES 10, Glucose 11, EGTA 0.5, 用KOH调节pH值至7.4. 记录IK的细胞外液为改良Tyrode液(mmol/L): NaCl 144, KCl 4.0, CaCl2 1.8, MgCl2 0.53, Na2HPO4 0.33, HEPES 5, Glucose 5.5, 用NaOH调节pH值至7.4. 电极内液为(mmoL/L): KCl 130, K2ATP 5.0, creatine phosphate 5.0, HEPES 5.0, 用KOH调节pH值至7.4. 记录ICRAC的细胞外液为(mmoL/L): NaCl 140, KCl 2.8, CaCl2 10, MgCl2 0.5, HEPES 10, Glucose 11, 用NaOH调节pH值至7.4. 电极内液为(mmoL/L): Potassium glutamate 145, NaCl 8.0, MgCl2 1.0, MgATP 0.5, EGTA 10, HEPES 10, 用CsOH调节pH值为7.2. Ber为黄色结晶粉末, Mr: 407.85, 纯度>95%, 购自宜昌制药厂. Collagenase I, HEPES, K2ATP, creatine phosphate, Potassium glutamate和MgATP均为Sigma公司产品. EGTA购自Fluka Biochemika公司.
采用改良的Seglen法 (Methods Cell Biol 1976; 13: 29-83). 成年Wistar大鼠(雌雄不限, 180±20 g, 由华中科技大学同济医学院实验动物部提供)采用30 g/L戊巴比妥钠50 mg/kg ip麻醉. 肝素抗凝, 行门静脉插管. 首先用无Ca2+, 无Mg2+的D-Hanks液灌流肝脏数分钟, 直至肝脏内残血排尽. 改用含I型胶原酶0.3 g/L D-Hanks液, 循环灌流10 min, 直至肝实质变软, 压之凹陷不易恢复, 肝包膜下组织呈"龟背状"裂隙为止. 灌流液均用O2饱和, 并保持37 ℃恒温. 完整剥离肝脏并置于4 ℃的D-Hanks液中撕碎, 制成混合肝细胞悬液. 细胞悬液经200目筛网过滤至离心管中, 500 r/min离心2 min, 去上清, 重复离心3次以除去非肝实质细胞. 细胞接种在盖玻片上, 并保存在4 ℃ KB液中, 供8 h内使用. 此方法可获得85%-95%活细胞. 有活性的肝细胞胞体在相差显微镜下透亮呈圆形, 细胞膜完整无损且边界清晰. 而无活性的肝细胞肿胀, 出现空泡及胞膜破损. 将盖玻片放入1 mL的灌流槽中, 置于倒置显微镜 (XD-1012B, 南京) 载物台上. 用细胞外液冲洗多余的组织碎片和未覆着在盖玻片上的细胞. 采用全细胞膜片钳技术, 在电压钳模式下记录电流. 实验在室温(20-22 ℃)下进行. 玻璃毛胚GG-17(南京六合泉水教学实验仪器厂)经微电极拉制仪(pp-83, Narishige, Japan)分两步拉制成尖端直径为1-1.5 m的电极, 充灌电极内液并浸入细胞外液后电阻为2-4 M 电极与膜片钳放大器(PC-II型, 华中科技大学)连接, 放大器通过12位A/D和D/A数据转换器(DA-1型, 华中科技大学)由计算机控制. 刺激产生和信号采集由Pclamp软件(IbbClamp32, 华中科技大学)控制. 电极入水后补偿液接电位, 然后再进行封接, 封接电阻达1 G以上, 补偿快电容. 破膜后调节慢电容补偿和串联电阻补偿(50%-80%), 以减少钳位误差和记录信号失真. 当记录电流稳定, 即电流幅度保持稳定时开始加药进行实验.
统计学处理 实验数据用mean±SD表示, 采用t检验进行统计学处理, P<0.05表示差异有显著性.
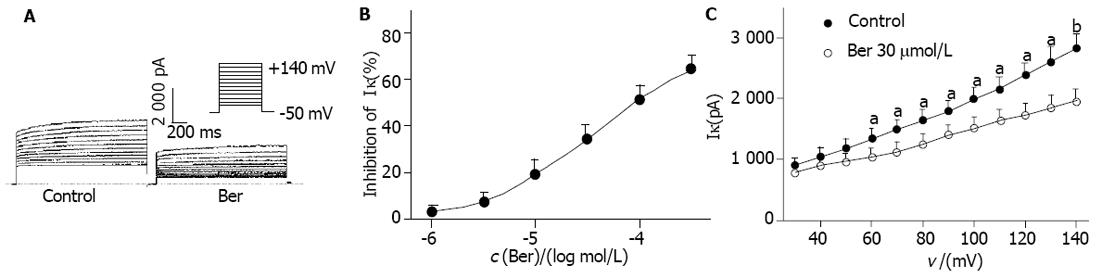
保持电位为-50 mV, 以0.1 Hz的频率, 施予+140 mV, 900 ms的除极化脉冲, 可引出一外向电流, 此电流可被TEA 8 mmoL/L阻滞, 即IK. 将测试脉冲末的电流幅值作为IK的幅值. Ber 10, 30 moL/L分别使IK由给药前的2 815±243 pA减小至2 273±202 pA和1 889±137 pA (抑制率分别为19.3%±2.7%和34.1%±6.0%). 1-300 moL/L浓度依赖性地抑制IK, 经Hill方程拟合后, EC50为39±5 moL/L, nH为0.82±0.05(图1B). 保持电位为-50 mV, 施予+30 ~+140 mV, 阶跃为10 mV, 900 ms的除极化脉冲, 记录IK, 并以各电流幅值对相应除极化膜电位作图得IK的I-V曲线(图1A, 1C). Ber 30 moL/L在膜电位为+60 ~+140 mV显著降低IK幅值, I-V曲线下移(n = 8, P<0.05 or P<0.01 vs control).
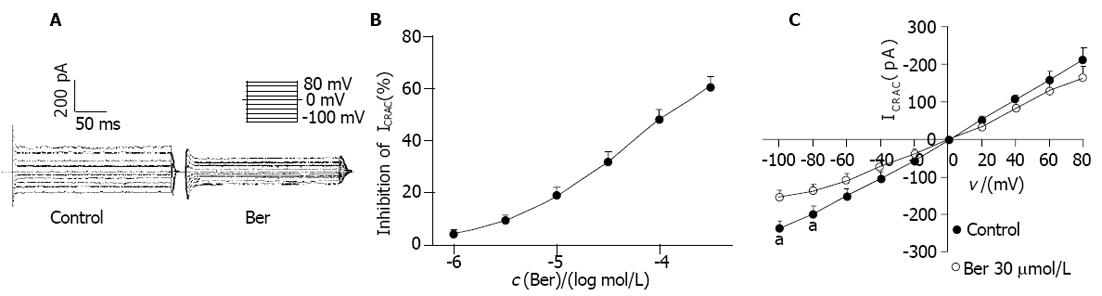
保持电位0 mV, 以0.2 Hz的频率, 施予-100 mV, 200 ms的脉冲刺激, 可记录到ICRAC. Ber 10, 30 moL/L分别使ICRAC由给药前的-238±22下降至-195±18 pA和-162±17 pA(抑制率分别为18.2%±3.5%和32.1%±4.0%). Ber 1-300 moL/L浓度依赖性地抑制ICRAC, EC50为47.2±10.9 moL/L, nH为0.71±0.09(图2B). 保持电位0 mV, 以0.2 Hz的频率, 施予-100 ~+80 mV, 持续时间为200 ms, 阶跃为20 mV的脉冲刺激, 得到一系列电流, 即ICRAC, 分别测量各膜电位下的ICRAC, 以各脉冲下电流幅值对相应膜电位作图得ICRAC I-V曲线(图2A, C), 其反转电位约为0 mV. Ber 30 moL/L使各膜电位下ICRAC幅值降低, 对内向电流和外向电流均明显减小, 尤其减小膜电位为-100~-80 mV时的ICRAC幅值, 但不改变I-V曲线的形状及反转电位(n = 8, P<0.05 vs control).
我们首次观察到Ber对大鼠肝细胞IK和ICRAC均有抑制作用. 许多非兴奋细胞膜的电学改变与其生理功能直接相关, 如瞬时外向钾电流调节血小板的黏附和聚集. 肝细胞是一种非兴奋性细胞, 其膜电位在调节肝脏代谢, 物质转运以及细胞容积等方面起重要作用, 如影响葡萄糖生成, 氨基酸转运和胆盐摄取速度等[4]. 膜电位主要由细胞内、外K+浓度及细胞膜对K+通透性所决定. 文献报道, 跨膜胆汁酸的转运与K+有关. 细胞内低K+时, 可使胆汁淤积[5-6]. 另外, 细胞K+平衡与维持肝细胞的水化状态和蛋白代谢亦密切相关. 肝脏缺血缺氧时, 肝细胞体积与K+电导增加, 导致细胞死亡[7-8]. Nietsch et al[9]研究表明改变K+通道活性所引起的膜电位改变可能是人肝细胞瘤凋亡的机制之一, 抑制K+通道可延迟肝细胞凋亡和死亡. Neveux et al[10]研究表明钾通道阻滞剂通过影响大鼠肝细胞的能量代谢而发挥保护作用.
Hwang et al[3]报道, Ber(0.5, 5 mg/kg, ip)对t-BHP诱导的大鼠氧化应激性肝损伤具有保护作用. 我们观察到, Ber浓度依赖性地抑制IK, 其剂量范围与Hwang et al的报道相似, 我们亦发现海葵毒素(anthopleurin-Q, AP-Q)能选择性地增大大鼠肝细胞IK, 该作用与其抗CCl4所致肝损伤有关[11]. 而本实验中表明Ber通过选择性地抑制大鼠肝细胞IK, 来拮抗t-BHP诱导的大鼠氧化应激性肝损伤. 这种矛盾现象主要是由于CCl4和t-BHP诱导肝损伤的作用机制不同. Ber对t-BHP诱导的肝损伤有保护作用, 而对CCl4诱导的肝损伤没有保护作用, 这与本课题组的实验结果相一致[12].
Ca2+内流主要包括3种方式: 依靠浓度梯度的被动扩散, 受体门控性钙通道和电压依赖性钙通道. 大鼠肝细胞不存在电压依赖性Ca2+通道, 但具有钙释放激活的钙通道, 在调节肝细胞膜内外Ca2+交换中起重要作用[13-15]. ICRAC能通过两种耗竭Ca2+库的方式激活. 一种为EGTA通过鳌合胞内Ca2+, 被动地耗竭Ca2+贮库, 缓慢激活ICRAC; 另一种为IP3通过与内质网IP3受体结合, 主动地耗竭Ca2+贮库, 迅速激活ICRAC[16-18]. 我们主要通过在电极内液中加入高浓度EGTA鳌合胞内Ca2+, 缓慢激活ICRAC[19].
Ca2+作为第二信使, 在肝细胞的糖原合成与分解、电解质转运, 以及肝细胞的生长增生过程中起着重要作用. 一旦细胞内外Ca2+失衡, 细胞将遭受功能性损伤, 甚至死亡[20-23]. 缺血再灌注肝损伤时, 肝细胞内Ca2+超载发生早, 明显先于肝细胞内其他生物化学改变, 很可能是重要的始动因素之一[24-26]. 肝细胞内Ca2+超载可激活磷脂酶和蛋白水解酶, 促进氧自由基大量生成, 使线粒体内ATP的合成减少, 是受损肝细胞死亡的最后共同通路, 也是细胞凋亡的关键因素之一[27-29]. 因此, 阻断肝细胞内Ca2+超载这一重要环节, 对于减轻肝缺血再灌注损伤, 保护肝功能, 具有重要的临床意义.
Ber抑制ICRAC的EC50为47.20 moL/L, 高于其抑制心肌细胞电压依赖性钙通道的EC50[30]. Ber对这两种电流的敏感性不同进一步说明ICRAC不同于电压依赖性的Ca2+通道. Ber通过抑制肝细胞IK, 减少钾离子外流, 有利于维持肝细胞内外离子环境的稳定, 调节肝脏的代谢. 另外Ber通过抑制肝细胞ICRAC, 阻滞Ca2+内流, 防止肝细胞内Ca2+超载, 从而发挥其肝保护作用.
编辑: N/A
| 1. | Li Y, Fu LY, Yao WX, Xia GJ, Jiang MX. Effects of benzyltetrahydropalmatine on potassium currents in guinea pig and rat ventricular myocytes. Acta Pharmacol Sin. 2002;23:612-616. [PubMed] |
| 2. | Li BX, Yang BF, Zhou J, Xu CQ, Li YR. Inhibitory effects of berberine on IK1, IK, and HERG channels of cardiac myocytes. Acta Pharmacol Sin. 2001;22:125-131. [PubMed] |
| 3. | Hwang JM, Wang CJ, Chou FP, Tseng TH, Hsieh YS, Lin WL, Chu CY. Inhibitory effect of berberine on tert-butyl hydroperoxide-induced oxidative damage in rat liver. Arch Toxicol. 2002;76:664-670. [PubMed] [DOI] |
| 4. | Roman R, Feranchak AP, Troetsch M, Dunkelberg JC, Kilic G, Schlenker T, Schaack J, Fitz JG. Molecular characterization of volume-sensitive SK(Ca) channels in human liver cell lines. Am J Physiol Gastrointest Liver Physiol. 2002;282:G116-G122. [PubMed] |
| 5. | Denk GU, Soroka CJ, Mennone A, Koepsell H, Beuers U, Boyer JL. Down-regulation of the organic cation transporter 1 of rat liver in obstructive cholestasis. Hepatology. 2004;39:1382-1389. [PubMed] [DOI] |
| 6. | Cho WK, Siegrist VJ, Zinzow W. Impaired regulatory volume decrease in freshly isolated cholangiocytes from cystic fibrosis mice: implications for cystic fibrosis transmembrane conductance regulator effect on potassium conductance. J Biol Chem. 2004;279:14610-14618. [PubMed] [DOI] |
| 7. | Carini R, Autelli R, Bellomo G, Albano E. Alterations of cell volume regulation in the development of hepatocyte necrosis. Exp Cell Res. 1999;248:280-293. [PubMed] [DOI] |
| 8. | Abrahamse SL, Van Runnard Heimel P, Hartman RJ, Chamuleau RAFM, Van Gulik TM. Induction of Necrosis and DNA Fragmentation during Hypothermic Preservation of Hepatocytes in UW, HTK, and Celsior Solutions. Cell Transplant. 2003;12:59-68. [PubMed] [DOI] |
| 9. | Nietsch HH, Roe MW, Fiekers JF, Moore AL, Lidofsky SD. Activation of potassium and chloride channels by tumor necrosis factor alpha. Role in liver cell death. J Biol Chem. 2000;275:20556-20561. [PubMed] [DOI] |
| 10. | Neveux N, De Bandt JP, Fattal E, Hannoun L, Poupon R, Chaumeil JC, Delattre J, Cynober LA. Cold preservation injury in rat liver: effect of liposomally-entrapped adenosine triphosphate. J Hepatol. 2000;33:68-75. [PubMed] [DOI] |
| 11. | Zhou HY, Wang F, Zhang KQ, Cheng L, Zhou J, Fu LY, Yao WX. Electrophysiological effects of anthopleurin-Q on rat hepatocytes. World J Gastroenterol. 2004;10:96-99. [PubMed] |
| 12. | Janbaz KH, Gilani AH. Studies on preventive and curative effects of berberine on chemical-induced hepatotoxicity in rodents. Fitoterapia. 2000;71:25-33. [PubMed] [DOI] |
| 13. | Gregory RB, Rychkov G, Barritt GJ. Evidence that 2-aminoethyl diphenylborate is a novel inhibitor of store-operated Ca2+ channels in liver cells, and acts through a mechanism which does not involve inositol trisphosphate receptors. Biochem J. 2001;354:285-290. [PubMed] [DOI] |
| 14. | Cheng JS, Lo YK, Yeh JH, Cheng HH, Liu CP, Chen WC, Jan CR. Effect of gossypol on intracellular Ca2+ regulation in human hepatoma cells. Chin J Physiol. 2003;46:117-122. [PubMed] |
| 15. | Yule DI, Straub SV, Bruce JI. Modulation of Ca2+ oscillations by phosphorylation of Ins(1,4,5)P3 receptors. Biochem Soc Trans. 2003;31:954-957. [PubMed] [DOI] |
| 16. | Gregory RB, Sykiotis D, Barritt GJ. Evidence that store-operated Ca2+ channels are more effective than intracellular messenger-activated non-selective cation channels in refilling rat hepatocyte intracellular Ca2+ stores. Cell Calcium. 2003;34:241-251. [PubMed] [DOI] |
| 17. | Gregory RB, Barritt GJ. Evidence that Ca2+-release-activated Ca2+ channels in rat hepatocytes are required for the maintenance of hormone-induced Ca2+ oscillations. Biochem J. 2003;370:695-702. [PubMed] [DOI] |
| 18. | Wissing F, Nerou EP, Taylor CW. A novel Ca2+-induced Ca2+ release mechanism mediated by neither inositol trisphosphate nor ryanodine receptors. Biochem J. 2002;361:605-611. [PubMed] [DOI] |
| 19. | Rychkov G, Brereton HM, Harland ML, Barritt GJ. Plasma membrane Ca2+ release-activated Ca2+ channels with a high selectivity for Ca2+ identified by patch-clamp recording in rat liver cells. Hepatology. 2001;33:938-947. [PubMed] [DOI] |
| 20. | Kim JS, Qian T, Lemasters JJ. Mitochondrial permeability transition in the switch from necrotic to apoptotic cell death in ischemic rat hepatocytes. Gastroenterology. 2003;124:494-503. [PubMed] [DOI] |
| 21. | Zhu LP, Yu XD, Ling S, Brown RA, Kuo TH. Mitochondrial Ca(2+)homeostasis in the regulation of apoptotic and necrotic cell deaths. Cell Calcium. 2000;28:107-117. [PubMed] [DOI] |
| 22. | Kim JS, He L, Qian T, Lemasters JJ. Role of the mitochondrial permeability transition in apoptotic and necrotic death after ischemia/reperfusion injury to hepatocytes. Curr Mol Med. 2003;3:527-535. [PubMed] [DOI] |
| 23. | Jaeschke H, Lemasters JJ. Apoptosis versus oncotic necrosis in hepatic ischemia/reperfusion injury. Gastroenterology. 2003;125:1246-1257. [PubMed] [DOI] |
| 24. | Silomon M, Pizanis A, Rose S. Oxyradical-mediated hepatocellular Ca2+ alterations during hemorrhagic shock and resuscitation. Shock. 1999;11:193-198. [PubMed] [DOI] |
| 25. | Sung YJ, Sung Z, Ho CL, Lin MT, Wang JS, Yang SC, Chen YJ, Lin CH. Intercellular calcium waves mediate preferential cell growth toward the wound edge in polarized hepatic cells. Exp Cell Res. 2003;287:209-218. [PubMed] [DOI] |
| 26. | Ben Abdennebi H, Steghens JP, Hadj-Aïssa A, Barbieux A, Ramella-Virieux S, Gharib C, Boillot O. A preservation solution with polyethylene glycol and calcium: a possible multiorgan liquid. Transpl Int. 2002;15:348-354. [PubMed] [DOI] |
| 27. | Jambrina E, Alonso R, Alcalde M, del Carmen Rodríguez M, Serrano A, Martínez-A C, García-Sancho J, Izquierdo M. Calcium influx through receptor-operated channel induces mitochondria-triggered paraptotic cell death. J Biol Chem. 2003;278:14134-14145. [PubMed] [DOI] |
| 28. | Lemasters JJ, Qian T, He L, Kim JS, Elmore SP, Cascio WE, Brenner DA. Role of mitochondrial inner membrane permeabilization in necrotic cell death, apoptosis, and autophagy. Antioxid Redox Signal. 2002;4:769-781. [PubMed] [DOI] |
| 29. | Nieminen AL. Apoptosis and necrosis in health and disease: role of mitochondria. Int Rev Cytol. 2003;224:29-55. [PubMed] [DOI] |
| 30. | Xu SZ, Zhang Y, Ren JY, Zhou ZN. Effects of berberine of L- and T-type calcium channels in guinea pig ventricular myocytes. Zhongguo Yao Li Xue Bao. 1997;18:515-518. [PubMed] |