修回日期: 2004-02-09
接受日期: 2004-02-24
在线出版日期: 2004-06-15
目的: 建立永生性大鼠肝星状细胞(hepatic stellate cell, HSC)系.
方法: 采用改良胶原酶原位灌注法消化肝脏, 经Nycodenz密度梯度离心分离、鉴定成年Sprague-Dawley大鼠的HSC. 然后通过细胞克隆建立HSC系(HSC-PQ)并传代培养. 结合细胞动力学、光镜、透射电镜及免疫细胞化学技术检测HSC-PQ系的倍增时间、a平滑肌肌动蛋白(a-smooth muscle actin, a-SMA)及结蛋白表达、细胞外基质(extracellular matrix, ECM)合成等表型特征.
结果: 分离获得的HSC约为2×107细胞/只大鼠, 细胞成活率>95%, 纯度>90%, 培养2 wk后几乎所有HSC均已活化. 在此基础上经细胞克隆所得的HSC-PQ系表型类似成纤维细胞, 倍增时间约75 h, 可表达结蛋白、a-SMA以及除IV型胶原外的多种ECM成分(I型胶原、III型胶原、纤维连接蛋白、层粘连蛋白等), 且传代过程中增生、ECM表达等特性均保持稳定. 该细胞系已在体外培养32代(1年以上), 提示其具有永生性.
结论: 已成功建立永生性大鼠HSC系, 该细胞系的基本特征与活化的原代HSC相似.
引文著录: 潘勤, 李定国, 汪余勤, 徐芹芳. 永生大鼠肝星状细胞系的建立及鉴定. 世界华人消化杂志 2004; 12(6): 1337-1340
Revised: February 9, 2004
Accepted: February 24, 2004
Published online: June 15, 2004
AIM: To establish and identify a novel immortalized rat hepatic stellate cell (HSC) line.
METHODS: Primary HSCs were isolated from the liver of adult male Sprague-Dawley rats by a combination of pronase-collagenase perfusion and density gradient centrifugation. Then a new HSC line, being HSC-PQ, was established, cultured, and passaged by way of cellular clone. Furthermore, cellular dynamics, light microscopy, transmission electron microscopy, and immunocytochemistry were employed to investigate characteristics of the HSC line.
RESULTS: About 2×107 HSCs could be harvested from a Sprague-Dawley rat with the live rate over 95% and purity over 90%. Afterwards, HSC-PQ line was obtained on the basis of total activation of primary HSCs. The phenotype of HSC-PQ cells resembled that of fibroblasts. Firstly, the existence of a-SMA as well as desmin in these cells exhibited their HSC-derived-myofibroblast identity clearly. Secondly, both the doubling time of about 75 hours, and the stable expression of extracellular matrixs including collagen type I, collagen type III, fibronectin, laminin, etc. showed the fibroblast-like-characteristics of HSC-PQ line. But collagen IV could not be detected in cytoplasm. In addition, maintaining over one year, 32 passages of the cell line might demonstrate its immortalisation.
CONCLUSION: We have established a new immortalized rat HSC line (HSC-PQ), which shares most of the characteristics with primary activated rat HSCs.
- Citation: Pan Q, Li DG, Wang YQ, Xue QF. Establishment and identification of a novel immortalized rat hepatic stellate cell line HSC-PQ. Shijie Huaren Xiaohua Zazhi 2004; 12(6): 1337-1340
- URL: https://www.wjgnet.com/1009-3079/full/v12/i6/1337.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v12.i6.1337
目前, 肝星状细胞(hepatic stellate cell, HSC)已被公认为肝纤维化形成的关键细胞, HSC活化是肝纤维化发生的中心事件[1-19,21-31]. 因而对HSC功能及活化机制的研究已成为肝纤维化研究的热点问题. 然而, 原代鼠源性HSC的分离方法繁琐、产量较低, 人类HSC来源困难, 现有HSC系的种类有限且不易获得, 这些都给研究工作造成很大困难. 为此, 我们尝试建立新的HSC系以代替原代HSC, 籍以满足科研工作对HSC的大量需求.
成年♂Sprague-Dawley大鼠2只(清洁级), 体质量400-500 g (中国科学院上海实验动物中心); IV型胶原酶、链酶蛋白酶E和Nycodenz (Sigma), 脱氧核糖核酸酶(华美), DMEM培养基(Gibco), a-SMA多克隆抗体、结蛋白多克隆抗体、I型胶原多克隆抗体、III型胶原多克隆抗体、纤维连接蛋白多克隆抗体和层粘连蛋白多克隆抗体(Boster); HL-3恒流泵(上海沪西仪器厂), CO2培养箱(Hirasaua Works), H-500型透射电子显微镜(Hitachi).
大鼠HSC分离采用改良Friedman法, 即经门静脉依次用无Ca2+灌流液、链霉蛋白酶E溶液、IV型胶原酶溶液灌流, 然后剪碎肝脏, 置于含链霉蛋白酶E、IV型胶原酶及脱氧核糖核酸酶的溶液中震荡30 min. 细胞悬液离心后洗3次, 再加入Nycodenz离心. 吸取表面的HSC, 重悬于DMEM培养液(含200 mL/L小牛血清)中. 采用细胞计数板计算细胞得率: 每毫升细胞数 = t×每大格平均细胞数×104×(1÷稀释倍数). 采用台盼蓝排斥试验判断细胞成活率; 在波长325-328 nm的紫外线激发下观察有荧光的细胞比率, 以计算HSC纯度. 然后, 将HSC悬液接种于培养瓶2 wk, 以使其完全活化. 待活化的HSC长满瓶(约80%)时置于紫外灯下照射1 h. 处理后的HSC以2.5 g/L胰蛋白酶-0.2 g/L EDTA溶液消化、传代, 并采取逐步稀释的方法接种于96孔培养板(密度依次为4, 2, 1/孔). 最终选取存活并形成克隆的细胞(HSC系)继续培养、传代. 同时将所得的HSC系细胞接种于24孔培养板(2×105/孔)并培养5 d. 期间每隔24 h取3孔, 计数后绘制生长曲线. 按下式计算细胞倍增时间(t为培养时间, No及Nt分别为接种后及培养t小时后的细胞数): 细胞倍增时间 = t×lg2÷(lgNt-lg Nt). 另以-20 ℃丙酮固定HSC系细胞30 min, 再分别滴加a-SMA、结蛋白、I型胶原、III型胶原、纤维连接蛋白及层粘连蛋白抗体50 mL (稀释倍数1:50-1:200), 置于37 ℃中孵育60 min, 随后顺序滴加正常兔血清, 生物素化二抗, 并采用DAB显色. 此外取1×107个HSC系细胞, 以20 g/L 4 ℃戊二醛固定2 h, 离心后再以10 g/L锇酸固定2 h, 经丙酮、乙醇逐级脱水, 环氧树脂包埋, 醋酸铀及柠檬酸铅染色, 超薄切片, 置于透射电镜下观察细胞超微结构.
统计学处理 均以量mean±SD表示, 采用单因素方差分析检验, 以SPSS软件进行处理.
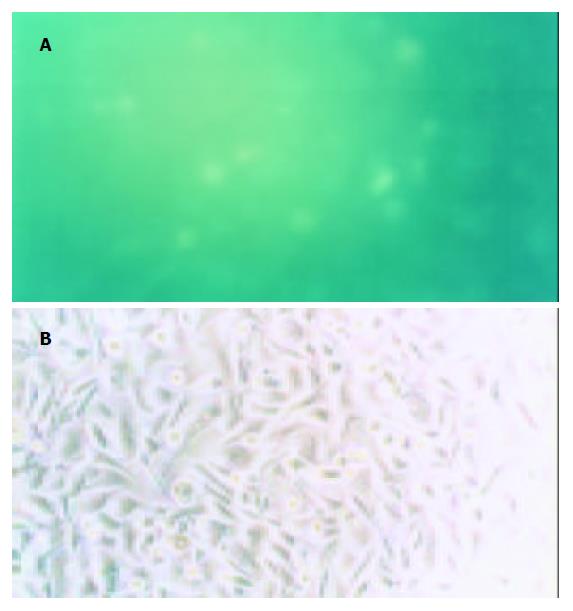
Friedman法, HSC得率约为2×107细胞/只大鼠. 经台盼蓝排斥实验观察, 细胞成活率>95%. 新分离的HSC呈圆形, 折光性很强, 绝大多数在激发波长325-328 nm的荧光显微镜下具有蓝绿色荧光, 纯度>90%(图1A). 接种24 h后部分HSC开始贴壁、生长. 48 h后细胞伸展已非常显著, 胞体变扁, 直径增加3-5倍, 呈现星形外观, 高倍镜下可见脂滴环绕在细胞核周围, 胞质内出现大量纤维束, 形成明显的细胞骨架. 72 h后绝大多数HSC已贴壁, 部分分化为椭圆形、梭形或多边形, 核/浆比例变小, 细胞突起宽而扁. 培养至2 wk时几乎全部HSC均分化为星形(图1B).
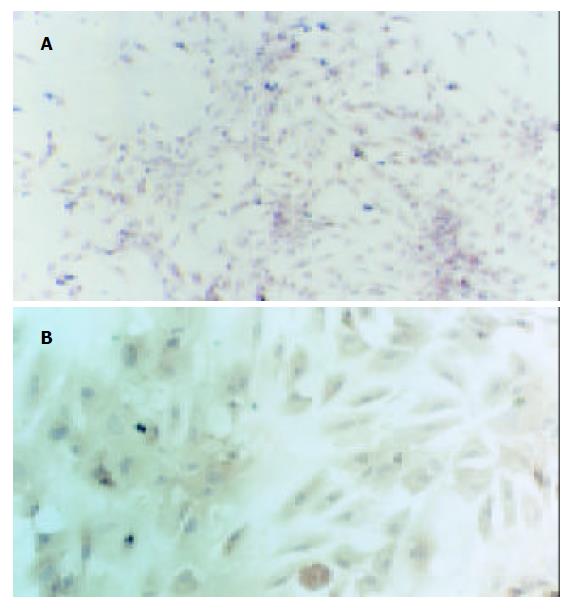
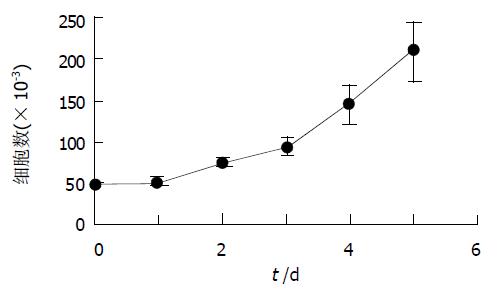
光镜下观察, HSC-PQ系类似成纤维细胞表型, 呈星形或梭形. 波长325-328 nm的紫外光激发下未出现自发荧光. 电镜下HSC-PQ系细胞均保持肌成纤维细胞的特征: (1)核/浆比例变小; (2)胞质突起长; (3)胞质周围出现大量应力纤维; (4)存在一至二个核仁; (5)粗面内质网扩张, 嵴的数量增加; (6)出现低密度、分散的核染色质; (7)脂滴消失. 免疫细胞化学检测显示, HSC-PQ系细胞 a-SMA、结蛋白染色均呈阳性(图2), 且胞质内表达I型胶原、III型胶原、纤维连接蛋白及层粘连蛋白. HSC-PQ系细胞生长速度较快, 倍增时间约75 h(图3).
体外培养32代(1年以上)后, HSC-PQ系细胞的结蛋白、纤维连接蛋白、层粘连蛋白、I型及III型胶原染色仍为阳性. 图像分析表明, 与建系初期相比, 此时HSC-PQ系细胞的细胞外基质(extracellular matrix, ECM)表达水平无显著差异. 相应的细胞倍增时间也保持在73 h左右.
激活的HSC是肝纤维化时产生各种ECM成分的主要细胞, 以及门静脉高压形成中的重要效应细胞[1-14,16-17,20], 也是抗纤维化治疗的主要靶细胞[15,18-19]. 以往实验多将活化的原代HSC作为研究对象. 然而HSC仅占肝脏非实质细胞数的5-10%或肝细胞数的3.6-6%, 因此不管采用何种分离技术, 最终得率仅能达到1×106-3×106/g [21-24]. 此外, 原代培养的HSC不甚稳定, 细胞分离状况及培养条件对实验结果的影响较大. 为克服上述缺陷, 目前逐渐倾向以HSC系代替原代HSC作为研究的靶细胞, 并已建立若干HSC系[21-30]: 如来源于大鼠的HSC系(NFSC, CFSC), 来源于小鼠的HSC系(A640-IS), 来源于人肝脏非实质细胞肿瘤的HSC系(L190), 质粒转染人肝脏肌成纤维细胞获得的HSC系(GREF-X)等. 不过, HSC在肝窦周围与其他细胞形成紧密连接, 从完整的肝脏中分离HSC并保证其具有足够的数量, 活力及纯度难度较大.另一方面, 在建立HSC系尤其是永生性HSC系的同时, 保持原代活化HSC的主要表型特征在技术上较为复杂. 人源性HSC也不易获得. 因而现有的HSC系种类仍然比较有限且不易获得, 有必要建立新的永生性大鼠HSC系. 为此, 我们首先在经典HSC分离方法[21-22]的基础上, 采取两大步骤获得原代大鼠HSC: (1)从肝组织中获取非实质细胞, 即采用链霉蛋白酶和胶原酶合并消化, 链霉蛋白酶能选择性破坏肝细胞, 减少肝细胞对HSC的黏附, 从而提高HSC的产量; (2)从肝脏非实质细胞中分离出HSC, 主要采用Nycodenz进行不连续密度梯度离心, 因HSC富含脂滴, 其平均密度在所有肝脏细胞中最低, 采用合适的密度梯度进行离心, 可使获得的HSC纯度达97%以上. 通过对二步法胶原酶原位灌注, 以及Nycodenz密度梯度离心技术的改良和优化, 实验中HSC的得率约为2×107细胞/只大鼠、纯度>90%, 成活率>95%. 该结果与以往文献[21-24]报道基本一致, 但操作较为简便易行. 通过连续培养, 原代大鼠HSC逐渐分化为星形, 核/浆比例变小, 突起伸长, 出现大量纤维束, 脂滴及维生素A荧光强度逐渐降低. 免疫细胞化学染色也显示, 胞质中结蛋白表达增加. 培养2 wk时a-SMA表达阳性, 显示此时HSC已由静止转为活化状态, 并转化为肌成纤维细胞[20]. 随后以常规传代、紫外线照射、逐步稀释等方法进行细胞克隆, 即获得一个HSC系(HSC-PQ). 该细胞系倍增时间约75 h, 形态类似成纤维细胞, 且在传代过程中均保持肌成纤维细胞的特征: (1)胞质突起长; (2)存在应力纤维; (3)有一至二个核仁; (4)粗面内质网扩张, 嵴的数量增加; (5)出现低密度分散的核染色质; (6)脂滴消失; (7)具有收缩性等[20]. 此外, 还可表达HSC特异性标志物结蛋白、HSC激活标志物a-SMA, 以及除IV型胶原外的多种ECM成分(I和III型胶原、纤维连接蛋白、层粘连蛋白等). 这表明HSC-PQ系细胞具有原代活化HSC的主要特性. 经体外32代(1年以上)的培养, HSC-PQ系的增生、ECM表达等诸多表型均保持稳定, 提示其已经具有永生性.
由此可见, HSC-PQ系与活化的原代HSC相似, 可用于HSC基因表达调控、抗纤维化药物评价及肝纤维化发病机制等领域的研究, 并将对HSC的细胞学研究, 特别是针对HSC的抗纤维化药物研究产生有力的推动作用.
| 1. | Martucci RB, Ziulkoski AL, Fortuna VA, Guaragna RM, Guma FC, Trugo LC, Borojevic R. Beta-carotene storage, conversion to retinoic acid, and induction of the lipocyte phenotype in hepatic stellate cells. J Cell Biochem. 2004;92:414-423. [PubMed] [DOI] |
| 2. | Montiel-Duarte C, Ansorena E, López-Zabalza MJ, Cenarruzabeitia E, Iraburu MJ. Role of reactive oxygen species, glutathione and NF-kappaB in apoptosis induced by 3,4-methylenedioxymethamphetamine ("Ecstasy") on hepatic stellate cells. Biochem Pharmacol. 2004;67:1025-1033. [PubMed] [DOI] |
| 3. | Fiorucci S, Antonelli E, Distrutti E, Severino B, Fiorentina R, Baldoni M, Caliendo G, Santagada V, Morelli A, Cirino G. PAR1 antagonism protects against experimental liver fibrosis. Role of proteinase receptors in stellate cell activation. Hepatology. 2004;39:365-375. [PubMed] [DOI] |
| 4. | Sakata R, Ueno T, Nakamura T, Ueno H, Sata M. Mechanical stretch induces TGF-beta synthesis in hepatic stellate cells. Eur J Clin Invest. 2004;34:129-136. [PubMed] [DOI] |
| 5. | Cardoso CC, Paviani ER, Cruz LA, Guma FC, Borojevic R, Guaragna RM. Effect of pentoxifylline on arachidonic acid metabolism, neutral lipid synthesis and accumulation during induction of the lipocyte phenotype by retinol in murine hepatic stellate cell. Mol Cell Biochem. 2003;254:37-46. [PubMed] [DOI] |
| 6. | Sung CK, She H, Xiong S, Tsukamoto H. Tumor necrosis factor-alpha inhibits peroxisome proliferator-activated receptor gamma activity at a posttranslational level in hepatic stellate cells. Am J Physiol Gastrointest Liver Physiol. 2004;286:G722-G729. [PubMed] [DOI] |
| 7. | Cao Q, Mak KM, Ren C, Lieber CS. Leptin stimulates tissue inhibitor of metalloproteinase-1 in human hepatic stellate cells: respective roles of the JAK/STAT and JAK-mediated H2O2-dependant MAPK pathways. J Biol Chem. 2004;279:4292-4304. [PubMed] [DOI] |
| 8. | Olaso E, Salado C, Egilegor E, Gutierrez V, Santisteban A, Sancho-Bru P, Friedman SL, Vidal-Vanaclocha F. Proangiogenic role of tumor-activated hepatic stellate cells in experimental melanoma metastasis. Hepatology. 2003;37:674-685. [PubMed] [DOI] |
| 9. | Kitamura Y, Ninomiya H. Smad expression of hepatic stellate cells in liver cirrhosis in vivo and hepatic stellate cell line in vitro. Pathol Int. 2003;53:18-26. [PubMed] [DOI] |
| 10. | Chen A. Acetaldehyde stimulates the activation of latent transforming growth factor-beta1 and induces expression of the type II receptor of the cytokine in rat cultured hepatic stellate cells. Biochem J. 2002;368:683-693. [PubMed] [DOI] |
| 11. | Uchio K, Tuchweber B, Manabe N, Gabbiani G, Rosenbaum J, Desmoulière A. Cellular retinol-binding protein-1 expression and modulation during in vivo and in vitro myofibroblastic differentiation of rat hepatic stellate cells and portal fibroblasts. Lab Invest. 2002;82:619-628. [PubMed] [DOI] |
| 12. | Nieto N, Friedman SL, Cederbaum AI. Stimulation and proliferation of primary rat hepatic stellate cells by cytochrome P450 2E1-derived reactive oxygen species. Hepatology. 2002;35:62-73. [PubMed] [DOI] |
| 13. | Tahashi Y, Matsuzaki K, Date M, Yoshida K, Furukawa F, Sugano Y, Matsushita M, Himeno Y, Inagaki Y, Inoue K. Differential regulation of TGF-beta signal in hepatic stellate cells between acute and chronic rat liver injury. Hepatology. 2002;35:49-61. [PubMed] [DOI] |
| 14. | del Carmen EM, Souza V, Bucio L, Hernández E, Damián-Matsumura P, Zaga V, Gutiérrez-Ruiz MC. Cadmium induces alpha(1)collagen (I) and metallothionein II gene and alters the antioxidant system in rat hepatic stellate cells. Toxicology. 2002;170:63-73. [PubMed] [DOI] |
| 15. | Kang LP, Qi LH, Zhang JP, Shi N, Zhang M, Wu TM, Chen J. Effect of genistein and quercetin on proliferation, collagen synthesis, and type I procollagen mRNA levels of rat hepatic stellate cells. Acta Pharmacol Sin. 2001;22:793-796. [PubMed] |
| 16. | Schwabe RF, Schnabl B, Kweon YO, Brenner DA. CD40 activates NF-kappa B and c-Jun N-terminal kinase and enhances chemokine secretion on activated human hepatic stellate cells. J Immunol. 2001;166:6812-6819. [PubMed] [DOI] |
| 17. | Gandhi CR, Uemura T, Kuddus R. Endotoxin causes up-regulation of endothelin receptors in cultured hepatic stellate cells via nitric oxide-dependent and -independent mechanisms. Br J Pharmacol. 2000;131:319-327. [PubMed] [DOI] |
| 18. | Wang LT, Zhang B, Chen JJ. Effect of anti-fibrosis compound on collagen expression of hepatic cells in experimental liver fibrosis of rats. World J Gastroenterol. 2000;6:877-880. [PubMed] [DOI] |
| 20. | Isono M, Soda M, Inoue A, Akiyoshi H, Sato K. Reverse transformation of hepatic myofibroblast-like cells by TGFbeta1/LAP. Biochem Biophys Res Commun. 2003;311:959-965. [PubMed] [DOI] |
| 21. | Shibata N, Watanabe T, Okitsu T, Sakaguchi M, Takesue M, Kunieda T, Omoto K, Yamamoto S, Tanaka N, Kobayashi N. Establishment of an immortalized human hepatic stellate cell line to develop antifibrotic therapies. Cell Transplant. 2003;12:499-507. [PubMed] [DOI] |
| 22. | Watanabe T, Shibata N, Westerman KA, Okitsu T, Allain JE, Sakaguchi M, Totsugawa T, Maruyama M, Matsumura T, Noguchi H. Establishment of immortalized human hepatic stellate scavenger cells to develop bioartificial livers. Transplantation. 2003;75:1873-1880. [PubMed] [DOI] |
| 23. | Sauvant P, Sapin V, Abergel A, Schmidt CK, Blanchon L, Alexandre-Gouabau MC, Rosenbaum J, Bommelaer G, Rock E, Dastugue B. PAV-1, a new rat hepatic stellate cell line converts retinol into retinoic acid, a process altered by ethanol. Int J Biochem Cell Biol. 2002;34:1017-1029. [PubMed] [DOI] |
| 24. | Schnabl B, Choi YH, Olsen JC, Hagedorn CH, Brenner DA. Immortal activated human hepatic stellate cells generated by ectopic telomerase expression. Lab Invest. 2002;82:323-333. [PubMed] [DOI] |
| 25. | Sakata R, Ueno T, Nakamura T, Sakamoto M, Torimura T, Sata M. Green tea polyphenol epigallocatechin-3-gallate inhibits platelet-derived growth factor-induced proliferation of human hepatic stellate cell line LI90. J Hepatol. 2004;40:52-59. [PubMed] [DOI] |
| 26. | Kariv R, Enden A, Zvibel I, Rosner G, Brill S, Shafritz DA, Halpern Z, Oren R. Triiodothyronine and interleukin-6 (IL-6) induce expression of HGF in an immortalized rat hepatic stellate cell line. Liver Int. 2003;23:187-193. [PubMed] [DOI] |
| 27. | Sauvant P, Abergel A, Partier A, Alexandre-Gouabau MC, Rock E, Sion B, Motta C, Sapin V, Azaïs-Bresco V. Treatment of the rat hepatic stellate cell line, PAV-1, by retinol and palmitic acid leads to a convenient model to study retinoids metabolism. Biol Cell. 2002;94:401-408. [PubMed] [DOI] |
| 28. | Segawa M, Kayano K, Sakaguchi E, Okamoto M, Sakaida I, Okita K. Antioxidant, N-acetyl-L-cysteine inhibits the expression of the collagen alpha2 (I) promoter in the activated human hepatic stellate cell line in the absence as well as the presence of transforming growth factor-beta. Hepatol Res. 2002;24:305. [PubMed] [DOI] |
| 29. | Cheng J, Imanishi H, Liu W, Iwasaki A, Ueki N, Nakamura H, Hada T. Inhibition of the expression of alpha-smooth muscle actin in human hepatic stellate cell line, LI90, by a selective cyclooxygenase 2 inhibitor, NS-398. Biochem Biophys Res Commun. 2002;297:1128-1134. [PubMed] [DOI] |
| 30. | Horie S, Kitamura Y, Kawasaki H, Terada T. Inhibitory effects of antisense oligonucleotides on the expression of procollagen type III gene in mouse hepatic stellate cells transformed by simian virus 40. Pathol Int. 2000;50:937-944. [PubMed] [DOI] |